Puff Bar Tests FDA Power Over Tobacco-Free Nicotine
- News This Week U.S. FDA
- March 3, 2021
- 3 minutes read
Credit: Puff Bar
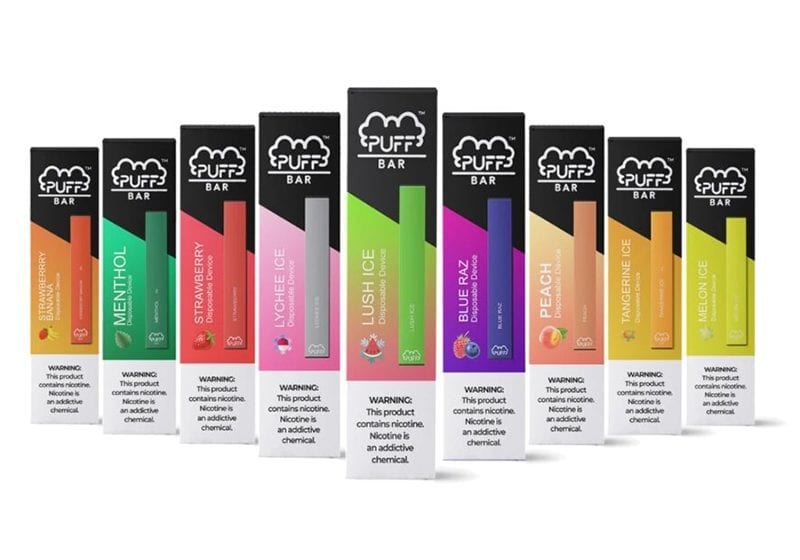
In July 2020, Puff Bar announced that it would cease all online sales and distribution in the U.S. until further notice. However, the brand resumed sales on its website last month with a changed product. To get around the ban, Puff Bar is now using tobacco-free nicotine, according to The Wall Street Journal.

The U.S. Food and Drug Administration (FDA) has started investigating Puff Bar’s redesigned fruit-flavored disposable vaporizers because of the changes, reports The Hill. The regulatory agency said it was aware of Puff Bar’s actions but would not say whether the agency’s ban was still applicable, citing the ongoing investigation.
In mid-2020, the FDA told Puff Bar to stop selling its fruity vaporizers as part of a broader crackdown on underage vaping. In a letter to Puff Bar, the FDA’s Center for Tobacco Products said Puff Bar products hadn’t been authorized for sale by the agency and that the company had made unauthorized claims on its website that its vaporizers were less harmful than traditional cigarettes.
In early 2020, the Trump administration banned fruity flavored e-cigarettes in reusable devices like Juul, the blockbuster brand that helped trigger the teen vaping craze in the U.S. Under that policy, only menthol and tobacco flavors were allowed for those devices. But the flavor ban did not apply to disposable vaping products like Puff Bar, which is offered in more than 20 flavors, including pina colada and pink lemonade.
Following the ban, Puff Bar quickly emerged as the vape of choice among young people. Puff Bar has fallen to No. 3 in the disposable category this year, after Bidi Stick and Blu, according to Nielsen data.
It’s unclear who owns Puff Bar. Previous owners include Cool Clouds Distribution in California and DS Technology Licensing in China.