Authorization Denied
- PMTA Regulation This Issue U.S. FDA
- November 1, 2021
- 19 minutes read

Credit: Waldemarus

The U.S. Food and Drug Administration devastates small businesses with a plethora of marketing denial orders.
By Timothy S. Donahue
At press time, the U.S. Food and Drug Administration had yet to approve an electronic nicotine-delivery system (ENDS) product for sale in the U.S. But it had killed much of the U.S. market for such products. As of Sept. 23, the agency had issued 323 marketing denial orders (MDOs) accounting for more than 1,167,000 flavored vaping products. In addition, the FDA previously refused to accept (RTA) or refused to file (RTF) a significant share of the nearly 7 million applications it received from more than 500 companies.
At least four lawsuits contesting MDOs have been filed in the 2nd, 4th, 6th and 11th Circuit Courts of Appeals against the FDA. Turning Point Brands (TPB) filed a petition for review with the United States Court of Appeals for the 6th Circuit. The petition forced the FDA to provide an administrative record for its decisions on PMTAs. TPB sells various flavored e-liquids marketed under the Solace, VaporFi and Vapor Shark brands.
In a surprise move as this magazine was going to press, the FDA rescinded Turning Point Brands’ MDO. The FDA admitted it made an error in TPB’s PMTA review and TPB did in fact submit studies that the agency decided during the PMTA process were needed, after saying for years the studies were not required. The FDA had not yet responded to the remaining cases as of press time.
“Upon further review of the administrative record, FDA found relevant information that was not adequately assessed,” the FDA letter to TPB states. “Specifically, your applications did contain randomized controlled trials comparing tobacco-flavored ENDS to flavored ENDS as well as several cross-sectional surveys evaluating patterns of use, likelihood of use, and perceptions in current smokers, current ENDS users, former tobacco users, and never users, which require further review.”
TPB was asking the court to review the FDA order “on the grounds that it is arbitrary and capricious, an abuse of discretion, contrary to the Federal Food, Drug and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act of 2009, and otherwise not in accordance with law.” The company requests the court “vacate or modify” the FDA order and asks that TPB be allowed to “continue to market the products subject to the challenged order.” Bidi Vapor filed a similar suit in the U.S. Court of Appeals for the 11th Circuit, BMF (Bad Modder Fogger) filed in the 4th Circuit and Magellan Technology, parent to DemandVape, has filed in the 2nd Circuit (those lawsuits are still active).
In addition to its arbitrary claim, Magellan also claims in its court petition that the “FDA’s issuance of an MDO in the absence of a finalized rule” setting forth the required contents of a PMTA is unlawful. “FDA’s adoption of a comparative efficacy standard for the granting of a marketing order for non-tobacco- and non-menthol-flavored ENDS products versus tobacco-flavored ENDS products is, in reality, a disguised tobacco product standard that has been adopted and is being applied by FDA through adjudication rather than adopted through notice-and-comment rulemaking,” states Magellan’s petition.
According to Mitch Zeller, the director of the FDA’s Center for Tobacco Products (CTP), many of the accepted applications ultimately received an RTF letter because they did not include required information. “For example, companies received RTF letters for not including required content such as ingredient listings, labels for each product to be marketed or adequate environmental assessments,” he wrote.
In a joint news release with Zeller and acting FDA Commissioner Janet Woodcock, the FDA explained that the applications from many MDO recipients “lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the levels of youth use” of ENDS products.
The PMTAs submitted by TPB and subsequently denied market access and the brought back under review by the FDA included an in-depth toxicological review, a clinical study and studies on patterns and likelihood of use, according to a motion to stay filed by TPB on Sept. 30. “In light of the unusual circumstances,” the FDA’s Center for Tobacco Products (CTP) Director Matt Holman stated in the letter. “FDA has no intention of initiating an enforcement action” against TPB’s products that had previously received an MDO.
Many of the current lawsuits against the FDA accuse the FDA of many of the same issues TPB’s withdrawn suit claimed. For example, TPB’s stay said the agency had moved the goalposts for data needed to receive a marketing order based on what the agency “learned” from the “review [of] PMTAs for flavored ENDS so far,” according to the stay. TPB noted that the “North Star of administrative law” is that agencies cannot induce regulated parties to rely on “agency representations about regulatory requirements” then penalize them using the previously unannounced criteria after the fact.
“But that is precisely what FDA did here,” the stay motion states. “[The] FDA reasoned that TPB failed to conduct ‘a randomized controlled trial and/or longitudinal cohort study’ or other studies performed ‘over time’ to show that TPB’s specific flavored products help adult users stop smoking more than tobacco-flavored products do. Yet FDA previously deemed these studies unnecessary.”
Tony Abboud, executive director of the Vapor Technology Association, suspects the FDA made an internal policy decision to change the PMTA standard to make it impossible after the fact for a company to comply and get a flavored ENDS application approved. “I think that that decision is being implemented application by application, which I don’t believe is fair under the law,” said Abboud. “I think that the refocusing on open system flavored e-liquids is a direct result of the public and political pressure that was placed upon the FDA by Congress, which expressly said they were trying to interfere with the regulatory process.”
What’s in a name?
Critics say the FDA has made several “sloppy” mistakes in reviewing PMTAs and issuing MDOs. Numerous companies say the agency was inconsistent in banning flavors based solely on the flavor’s name. Bidi Vapor’s parent, Kaival Brands, said that the agency banned its “Arctic” flavor, misidentifying it as a “not-menthol” flavor. TPB also says in its stay motion that the FDA is forcing TPB to pull nonflavored products from the market; however, the FDA’s order applies to “Authentic Tobacco” and “Bold Tobacco” yet not “Classic Tobacco” (which the FDA is still considering).

“Those are the same flavors with the same formulations; they just use different names across product lines. The same goes for ‘Ripe Tobacco’ (forbidden) and ‘Smooth Tobacco’ (reprieve) and for ‘Mint’ (banned) and ‘Mighty Menthol’ (allowed for now),” the stay explains. “It is anyone’s guess why some of these products must exit the market immediately yet others might pass muster if FDA actually reviews TPB’s studies.”
Since January 2021, the agency has issued at least 170 warning letters to firms that collectively have listed more than 17 million ENDS products with the FDA and that did not submit premarket tobacco product applications (PMTAs) for the products by Sept. 9, 2020. Applications for products manufactured by major companies, such as Vuse, Juul, Logic and blu, are still under review. During this time, the agency also granted substantial equivalence (SE) status (marketing approval) to over 350 combustible products from the cigar, pipe and hookah tobacco product categories.
Amanda Wheeler, president of the American Vapor Manufacturers Association (VMA) and the owner of Jvapes e-liquids (see “No Surrender,” page ?), assisted more than 230 small-sized to mid-sized e-liquid manufacturers in submitting PMTAs for more than 1.7 million products. Nearly all of those applications received either an RTA, RTF or an MDO.
Wheeler tweeted on Sept. 9 that it was a “tough day” for the industry because “lots of very good people who I respect deeply and who helped thousands of smokers quit got told by our government that their products were illegal. To all of you, I am so very sorry. To your customers, I am even more sorry. Our government is wrong on this.”
Before the announcement, many industry experts said that banning most e-cigarettes from the market could harm public health. In a commentary published on the Reason Foundation’s website, Guy Bentley, the organization’s director of consumer freedom research, states that the sooner that U.S. public health officials embrace vaping’s potential to improve public health by reducing smoking and smoking-related deaths, “the better off we’ll all be.” The result of shutting down a vast portion of the vape industry, he warns, will be more smoking.
Anti-vaping activists, by contrast, argued for a ban on e-cigarettes. In a recent blog post, Laurie Rubiner, executive vice president of domestic programs at the Campaign for Tobacco-Free Kids, and Linda Mendonca, president of the National Association of School Nurses and an assistant professor at the Rhode Island College School of Nursing, wrote that the “evidence is clear” that as long as any flavored e-cigarettes remain on the market, kids will get their hands on them (no reference to evidence was provided).
“To truly protect kids and end the youth e-cigarette epidemic, the FDA must eliminate the flavored and high-nicotine products—including the popular menthol flavor—that have driven this crisis,” the pair write. “Parents, educators and health advocates are counting on the FDA to take them off the shelves.”
Tom Miller, attorney general for the state of Iowa, said the FDA actions against flavors endanger public health. He said that the best science available indicates that most youths are not getting e-cigarettes from vape shops and that a significant number of adults are using products from vape shops to move away from combustible cigarettes.
“Let’s not forget the overwhelming risk to public health: The CDC [U.S. Centers for Disease Control and Prevention] estimates the burden of tobacco use in the United States is 480,000 lives a year, all of which is due to the use of cigarettes,” Miller said in a statement. “We believe in the strong, science-based regulation of alternative tobacco products, and the FDA is the best agency to undertake that task. Policymakers must strike the right balance between making accessible potentially lifesaving lower risk nicotine products while discouraging use by those who wouldn’t smoke, especially youth.”
Impacts of regulation
Several studies have suggested that if vape product sales were restricted to tobacco flavors, many would return to combustible tobacco. One study found that approximately one-third of U.S. vapers aged 18 to 34 say flavor bans would push them back to smoking traditional cigarettes. The study published in Nicotine & Tobacco Research analyzed data from February to May 2020 and looked at 2,159 young adults in Atlanta, Boston, Minneapolis, Oklahoma City, San Diego and Seattle, examining support for e-cigarette sales restrictions and the perceived impact of flavor and vaping bans.

Two other recent studies showed similar results. A study in JAMA Pediatrics showed that following San Francisco’s flavor ban, teens were more likely to smoke than those in other school districts. A different study in Nicotine & Tobacco Research shows that teens who vape would be smoking cigarettes if vapes hadn’t become available.
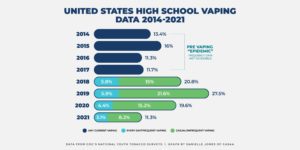
Recent evidence also seems to show that the overall youth use of e-cigarettes in the U.S. is declining. According to the 2021 National Youth Tobacco Survey (NYTS), the FDA and the CDC found that youth use of e-cigarettes fell sharply in 2021. It’s the second consecutive year of major declines. As is typical in the release of the NYTS data every year, media reports about the NYTS were all over the board. One headline read, “Big Drop in U.S. Teen Vaping with Covid Closures” while another read, “Teen Vaping Craze Shows No Sign of Slowing.”
The study shows that an estimated 11.3 percent (1.72 million) of high school students and an estimated 2.8 percent (320,000) of middle school students reported current e-cigarette use, lower than the 19.6 percent (high school) reported in 2020 and substantially lower than the 27.5 percent (high school) reported in 2019, according to previous FDA statements. Middle school vaping fell to 2.8 percent this year from 4.7 percent in 2020—a 40.4 percent decline. Middle school past 30-day vaping in 2020 fell 55.2 percent from 2019.
Chris Allen, chief scientific officer at Broughton, a contract research organization (CRO) delivering analytical, scientific and regulatory services for the ENDS industry, said that the FDA might well be using the NYTS to justify the “flurry of MDOs” issued for flavored e-liquids. He also said the majority of the companies that have fallen foul of the recent MDOs are responsible manufacturers supporting tobacco harm reduction.
“I completely accept that youth use is unacceptable; however, the issue doesn’t appear to lie primarily in open systems but a product that is currently outside the jurisdiction of FDA: a disposable containing synthetic nicotine,” Allen said. “Regardless of the product, or the source of nicotine, there’s no place for irresponsible marketing and distribution practices that keeps adding fuel to this fire. I fear that the latest action is simply going to lead to a seismic shift into the black market and unregulated (synthetic nicotine) products, which will be near on impossible for the U.S. government to control. From my personal perspective, this doesn’t seem an appropriate way to support THR [tobacco harm reduction].”
Industry representatives predict major battles at the state level. “States are just going to ban the sale of any non-FDA approved product,” said a vape shop owner, who asked not to be identified as he had not yet received an MDO. “This is just going to be a never-ending stream of court battles. I hope every company is at least considering appealing the MDO decisions. The whole PMTA process was a giant bait-and-switch.”
Manufacturers that submitted their applications by the Sept. 9, 2020, deadline but who have not yet received an MDO can effectively continue to sell their products as no ruling has been made on them; however, the FDA has made it clear that any company that does continue to sell these products will be doing so unlawfully, although they are not likely to face any enforcement action due to the agency’s limited resources.
Numerous companies are appealing their MDOs. Many are appealing MDOs they believe were wrongly issued because the PMTAs were for tobacco and/or menthol flavors. The AVM is helping its member companies file formal appeals with the FDA because the agency “in their sloppy haste, FDA not only threw out flavored products. They also threw out many [companies] [regular] tobacco and menthol flavors. We’re starting with some of those appeals specifically for what we feel were sort of administrative errors with tobacco and menthol and also working on broader appeals.”
Companies can also contact the CTP’s Office of Small Business Assistance (OSBA) with general questions regarding statutory and regulatory requirements, including the appeals process. Another option is the FDA Office of the Ombudsman, the agency’s “focal point for addressing complaints and assisting in resolving disputes between companies.”
Deanna Clark with the Clark-Esposito Law Firm stated in a blog post that each company must submit its own submission appealing the FDA decision. Companies should not send in an appeal combined with other companies, she cautioned. “Next, you want to address arguments refuting [the] FDA’s basis for your denial. It can’t just be where you’re complaining about how it’s unfair and the government sucks,” said Clark. “You need to use some rational basis behind what you’re submitting to them. And thirdly, you need to submit it to the right office and make sure it gets to the right people within the right timeframe.”
The e-cigarette saga with the FDA is far from over. Between lawsuits and appeals, many decisions may eventually be left out of the hands of the FDA entirely. The FDA’s ombudsman and appeals court judges could now decide the fate of flavored e-liquids. Congress could possibly step in and change the statutes, but many have said that is unlikely. The industry is also still waiting for decisions on the PMTAs filed by the major tobacco companies, and if anyone is approved, it may open the door for standard equivalency products. The only thing that hasn’t changed in the vaping industry is its uncertain future.
