Today, the U.S. Food and Drug Administration authorized the marketing of 22nd Century Group Inc.’s “VLN King” and “VLN Menthol King” combusted, filtered cigarettes as modified risk tobacco products (MRTPs). The FDA has not yet granted a MRTP to a vaping product, even though the agency has said e-cigarettes are less harmful than combustible cigarettes. Many experts have said the low-nicotine cigarettes from 22nd Century will actually cause people to smoke more cigarettes.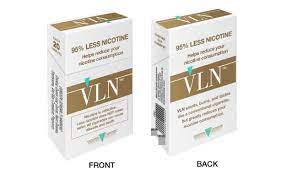
These are the first combusted cigarettes to be authorized as MRTPs by the agency and the second tobacco products overall to receive “exposure modification” orders, which allows them to be marketed as having a “reduced level of, or presenting a reduced exposure to,” a substance, according to a press release.
“Our mission is to find ways to stop tobacco-related disease and death. We know that three out of four adult smokers want to quit and the data on these products show they can help addicted adult smokers transition away from highly addictive combusted cigarettes,” said Mitch Zeller, director of the FDA’s Center for Tobacco Products. “Having options like these products authorized today, which contain less nicotine and are reasonably likely to reduce nicotine dependence, may help adult smokers. If adult smokers were less addicted to combusted cigarettes, they would likely smoke less and may be exposed to fewer harmful chemicals that cause tobacco-related disease and death.”
The exposure modification orders specifically authorize the manufacturer to market “VLN King” and “VLN Menthol King” with certain reduced exposure claims regarding nicotine, including:
“95% less nicotine.”
“Helps reduce your nicotine consumption.”
“…Greatly reduces your nicotine consumption.”
When using any of the reduced exposure claims in the product label, labeling or advertising, the company must include, “Helps you smoke less.” The FDA also recommends that the labeling and advertising include the statement, “Nicotine is addictive. Less nicotine does NOT mean safer. All cigarettes can cause disease and death.”
Despite today’s action, these products are not considered safe or “FDA approved.” There are no safe tobacco products, so people, especially young people, who do not currently use tobacco products should not start using them or any other tobacco product, according to a press release. The exposure modification orders do not permit the company to make any other modified risk claims or any express or implied statements that convey or could mislead consumers into believing that the products are endorsed or approved by the FDA, or that the FDA deems the products to be safe for use by consumers. These orders do not allow the company to market these products with therapeutic or cessation claims.

