
Qnovia announced positive results from its first in-human study of QN-01, an inhaled smoking cessation therapy that is currently being evaluated by the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) and the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA).
“Today marks a pivotal milestone for Qnovia as we believe this data validates the clinical translation of our platform for the first time in patients,” said Qnovia CEO Brian Quigley.
“One of the reasons that quitting smoking is so challenging is that cigarettes provide a significant nicotine spike directly into the bloodstream within seconds. The challenge with current nicotine replacement therapies is that they fail to deliver nicotine quickly enough and at concentrations high enough to effectively alleviate a smoker’s withdrawal symptoms. Unfortunately, this leads to smokers relapsing, resulting in a significant unmet need for novel smoking cessation therapies.

We believe our nicotine delivery platform has the potential to solve this complex pharmacokinetic puzzle and could be a game changer in alleviating cravings and withdrawal symptoms for those smokers who need it most
Brian Quigley, CEO, Qnovia
“We believe our nicotine delivery platform has the potential to solve this complex pharmacokinetic puzzle and could be a game changer in alleviating cravings and withdrawal symptoms for those smokers who need it most,” said Quigley.
“We are delighted to share that QN-01 demonstrated a superior pharmacokinetic profile compared to an existing inhaled NRT and was well tolerated. We look forward to submitting our IND and CTA for QN-01 and advancing our clinical development program into Phase 1/2 clinical trials next year.”
The Phase 1 study was an open-label trial evaluating the delivery of QN-01 for three different nicotine dosing regimens to determine the pharmacokinetics and safety profile of Qnovia’s drug delivery platform in 12 healthy adults who currently smoke combustible cigarettes. Each adult received three different doses of treatment delivered on subsequent days after a washout period.
The study confirmed dose-dependent pharmacokinetics of QN-01 delivered with the Qnovia’s RespiRx device. The mean maximum plasma concentration (Cmax) was higher, and the time to achieve the maximum plasma concentration (Tmax) was lower for QN-01 across all three dose regimens, demonstrating superior pharmacokinetics compared to an existing inhaled nicotine replacement therapy (NRT). The drug-device combination was well tolerated with no severe adverse events and few minor adverse events typical of inhaled nicotine.
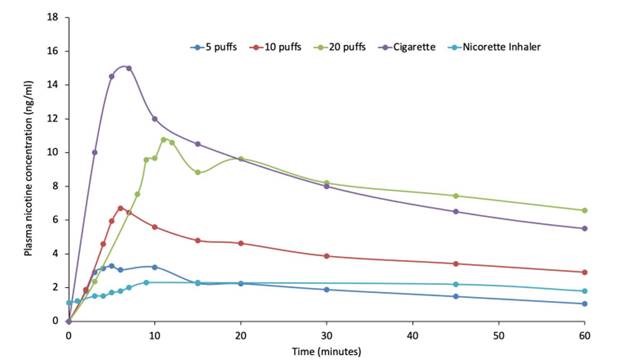
“We are pleased to see our platform being utilized for the first time in human clinical trials. We believe these results validate that we have identified an optimal Cmax for QN-01 that is higher than the currently available inhaled NRTs but lower than combustible cigarettes, thus enabling QN-01 to have optimal therapeutic efficacy while mitigating abuse liability potential,” said Mario Danek, Founder and chief technology officer of Qnovia.
“What makes our platform unique is that the RespiRx device utilizes a vibrating mesh nebulizer aerosol engine with zero heat to create an aerosol that can be inhaled by the smoker. Given our e-liquid drug product is not heated, there is no formation of thermal degradants or other toxicants during the aerosol generation process. As a result, our device platform is uniquely positioned to meet CDER’s safety standards. Looking ahead, we are committed to advancing QN-01 into the next stage of clinical development and will be working closely with FDA and MHRA to bring this treatment option to the millions of smokers who want to quit.”

