
The concept of tobacco harm reduction is gaining momentum in Europe, according to a new report by The European Tobacco Harm Reduction Advocates (ETHRA). On July 8, ETHRA published the results of its 2020 EU Nicotine Users Survey.
Launched online by ETHRA in the last quarter of 2020, the questionnaire addressed consumer use of nicotine products. Topics included smoking and the desire to quit, use of safer nicotine products and barriers to switching caused by European and national regulations. More than 37,000 people, including more than 35,000 EU residents, participated in the ETHRA survey.
According to ETHRA, more than 27,000 of the survey participants had completely quit smoking. Vapes, snus and nicotine pouches are the main harm reduction products used to quit. Among the respondents who had ever smoked, 83.5 percent of vapers and 73.7 percent of snus users had successfully stopped smoking.
Over 93 percent of vapers and 75 percent of snus users cited harm reduction and improvements to health as their reasons for adopting these products. The report shows that the reduced cost compared to smoking, the availability of flavors, the availability of products and the ability to adjust vaping products are other major factors for consumers when switching to harm reduction products.
The lack of availability of low-risk nicotine products presents a major obstacle to consumers wishing to quit smoking.
ETHRA
However, smoking remains the predominant way of consuming nicotine in Europe. More than 67 percent of the current smokers who responded to our survey want to quit, but the ETHRA report shows they face barriers in their desire to be smoke-free.
The lack of availability of low-risk nicotine products presents a major obstacle to consumers wishing to quit smoking. The EU ban on the sale of snus (which exempts Sweden), illustrates this barrier, with 31 percent of current smokers indicating that they would be interested in trying snus if its sale were legalized in the EU.
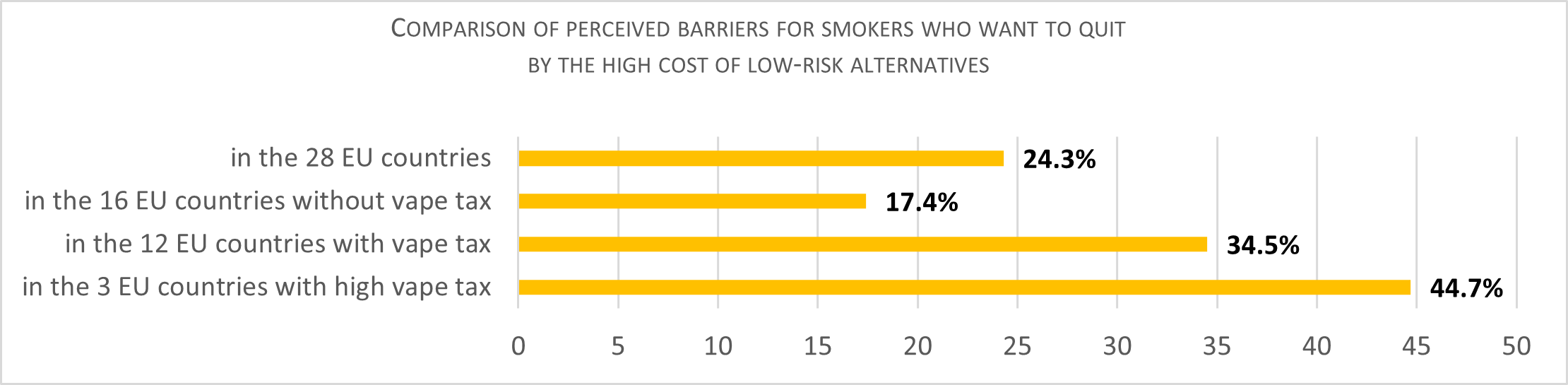
A quarter (24.3 percent) of those who smoke but who want to quit cited the high price of safer alternatives as a barrier to quitting smoking. This number rises to 44.7 percent in countries with a high tax on vaping products, such as Estonia, Finland and Portugal.
The EU Tobacco Product Directive (TPD) restrictions of a maximum nicotine concentration of 20mg/ml and a maximum bottle volume of 10ml have driven vapers to very low nicotine e-liquids. More than 30 percent of people who vape and smoke (“dual users”) believed they could completely quit smoking if the EU nicotine limit were increased.
Meanwhile, harm reduction advocates are anxiously awaiting pending amendments to the TPD. If the EU bans flavors, 28 percent of vapers are likely to restart smoking, and 71 percent would consider using the black market or other alternative sources, according to the survey. In the 16 EU countries without a vape tax, only 1 percent of vapers are currently using alternative sources.
If the EU repealed the 10 ml bottle limit, 89 percent of vapers said they would buy larger bottles of e-liquid to reduce plastic waste. 83 percent of vapers are in favor of having access to an EU database on e-liquid ingredients.
Considering the results from the EU Nicotine Users Survey 2020, ETHRA recommends the lifting of the EU ban on the sale of snus, revising upwards the 10 ml refill bottle and 20 mg/ml nicotine concentration limits, and the publication of databases on vaping products.
The organization also urges a repeal of vaping taxes in 12 countries and the lifting of flavor bans in Estonia, Finland and Hungary to give European smokers the freedom to quit smoking using low-risk products.






















