Vapers say taste matters. According to a survey conducted by Frost & Sullivan, among 3,000 Chinese vapor consumers, taste was a key factor in choosing an e-cigarette. The top three indexes in flavor were the overall sensation of taste (66 percent), aroma (61 percent) and the amount of vapor (50 percent).
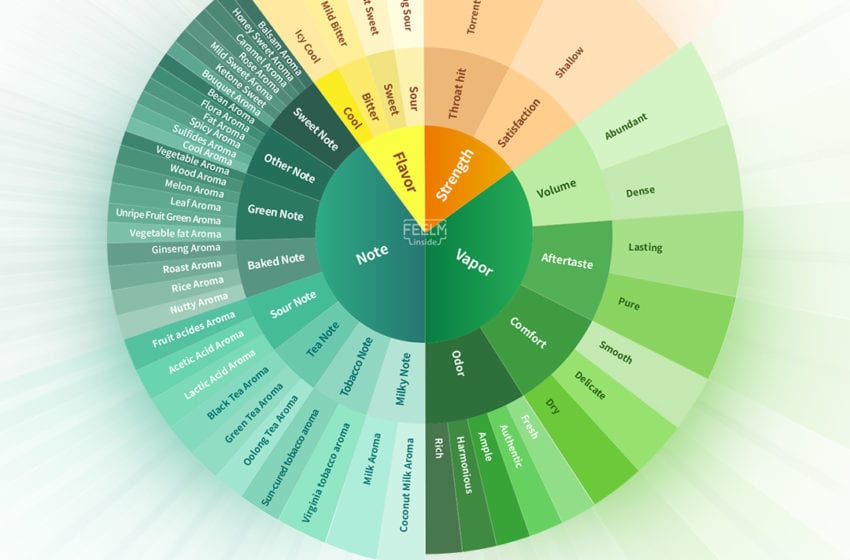
In late-December, FEELM, a heating technology brand, introduced the industry’s first Taste Evaluation Model. The model allows FEELM researchers the ability to describe the taste of atomization scientifically. Composed of four dimensions, flavor, strength, note and vapor, and 51 specific indexes, the model establishes a system to evaluate the human senses of mouth, tongue, nose and throat.

Frank Han, CEO of FEELM, said the company’s devotion to continuously improving the taste of e-cigarettes originated from a client’s concern. “How’s the taste of California strawberries at 6am? What’s the difference between it and that of refrigerated ones?” he said the client asked. “Could FEELM ceramic coils bring back the taste of California strawberry at 6am with flavored e-liquid?”
Inspired by the client, FEELM team members conducted numerous studies with the goal of developing a scientific system to properly evaluate and provide the perfect flavor. “As you can imagine, analyzing and delivering such an abstract concept as taste will [take] anyone tons of effort,” Han said.
In order to help with challenging endeavor, FEELM’s parent company, Smoore Technology, set up several research institutes both in China and other locations around the world (Smoore does not produce any e-liquid). More than 700 Smoore technical experts that have received more than 2,000 international patents began building a world-leading atomization platform, according to the release. Now, over 75 percent of those researchers are focused on understanding and improving taste through atomization. Smoore also established relationships with other research institutions such as Tongji University, Tsinghua University and Princeton University.

“This institute will focus on the background study and harm reduction from new perspectives such as bio-medicine and artificial intelligence,” the release states. “To further study the science behind atomization, FEELM has also established a professional taste evaluation team, and built a Taste Evaluation Lab complied with the national standard and could hold seven tasters at a time.”
The process for evaluating a flavor is precise. Before picking up a vaping device, each taster washes and sanitize their hands and then chews a slice of lemon or yellow peach to clean the palate. Testers than drink boiled water to freshen up the mouth and smell coffee beans, according to a FEELM representative.

“It is not until the light and the exhaust fan in the booth are turned on that the taster begins tasting. While tasting, a recorded form with all detailed criteria is prepared for scoring and taking notes,” the representative said. “Afterwards, the same vaping device will be handed over to chromatography, electron microscope as well as reliability labs to analyze its e-liquid ingredients, coil and safety … a diagnostic report will be produced based on both human and machine criteria. FEELM is then able to figure out the deficiencies in the vaping experience and work out precise solutions.”

The FEELM Taste Scientific Institute is made up of more than 10 labs, including Cell Lab, Taste Evaluation Lab, Element Analysis Lab, Chromatography Lab, and Electron Microscope Lab. The company says its labs can produce 50 physical tests, more than 200 chemical tests, six microbiological test items, three genetic toxicology tests, three categories of clinical abuse tests, as well as three categories of human factor testing.
“Besides scientific research, great taste cannot be separated from production. Smoore has established a rigorous production safety standard, which is even stricter than the TPD in the EU and AFNOR in France,” the representative said. The production protocols are also more stringent than what is required by the premarket tobacco product application (PMTA) process for the U.S. Food and Drug Administration (FDA), covering 50 individual tests.”
FEELM also developed the first fully automated pod production line in the vapor industry. Its production capacity is 6,000 pieces per hour, boosting efficiency five times over manual production. Automated production guarantees the stability and consistency of quality.
“Taste is a meaningful word,” said Han. “Great taste relies on the scientific system of fundamental research, the persistent improvement of product development and manufacturing, strict quality control, and a devoted focus towards science and ingenuity.”