
The UKVIA forum offered insight into the most significant threats to the U.K. vaping industry.
By George Gay
The U.K. vaping industry, which has benefited from some progressive government policies in the past, nevertheless found itself with the sword of Damocles hanging over its head as participants met in London for the annual forum of the U.K. Vaping Industry Association (UKVIA) on Nov. 10. And I am not being overly dramatic here.
As part of his forum presentation, the Conservative Member of Parliament Adam Afriyie warned attendees that the U.K. “could go the way of Australia in the blink of an eye”—and he wasn’t talking galahs and wombats; he was referring, I assume, to a generally very restrictive vaping environment, though not necessarily to the specific prescription-based medicalization of vaping in that country.
What was at the back of everybody’s mind on Nov. 10 was a government consultation on smoking and vaping issued in October and due to end on Dec. 6, which made the theme of the forum, “Accelerating action: Securing a world without smoking,” something of an object lesson in being careful what you wish for.
The consultation includes a proposal to make it an offense to sell any product containing tobacco to those born on or after Jan. 1, 2009, which would raise the legal smoking age by a year annually until it applies to the whole population. The government claims this has the potential to almost completely phase out tobacco smoking among young people by 2040.
Worryingly for the vaping industry, some might suggest this policy would be a way of single-handedly ensuring a U.K. without tobacco smoking, eliminating the need for products that can substitute for combustible cigarettes, such as vapes. And for those wedded to the idea that vapers should be drawn only from the ranks of smokers, it would mean the vaping industry had a limited future, one where, within a predictable timeframe, it would simply be managing decline.
On the other hand, some might argue that with one of the UKVIA’s goals being to support the government in reaching its 2030 smoke-free target, and given the government’s claims about the damage caused by tobacco smoking being so alarming, timing is of the essence and that vaping can help things along, though the idea that it is necessary to act speedily is perhaps undermined by the facts that the risk of cigarette smoking has been known about for more than half a century, and substitute products have been around for even longer.

On the other hand, if I haven’t run out of hands, the future might not look this bleak because there is the usually unspoken argument, with which I would agree, that vapers need not be recruited only from the ranks of smokers. We must be grown up and realize that some people will, for the foreseeable future, seek recreational drugs, and nicotine delivered through vaping must be a better, less risky candidate than many others on offer, especially alcohol, which surely must be the subject of the next government consultation.
But I digress. There are proposals in the consultation that forum participants will likely have considered to be more directly threatening than a creeping tobacco smoking ban. “The U.K. government and devolved administrations [those in Northern Ireland, Scotland and Wales] have a duty to protect our children from the potential harms associated with underage vaping while their lungs and brains are still developing,” the consultation says in part. “So, the U.K. government and devolved administrations are consulting on several proposals on youth vaping, including restricting flavors, regulating point-of-sale displays, regulating packaging and presentation, considering restricting the supply and sale of disposable vapes, whether regulations should extend to non-nicotine vapes and taking action on the affordability of vapes.
What I find a little concerning about the above is that, unless the consultation document was put together by people who started messing with nicotine before their brains had fully developed, it seems to have been written in something of a rush. The fourth proposal is apparently about “consulting on … considering restricting,” which seems a mite convoluted. And the fifth proposal goes into passive mode whereby the regulations might extend, seemingly of their own accord. That is creepy.
These might seem to be minor semantic matters, but I’m not sure they are because they reflect on how much time and effort went into producing the consultation document and, more worryingly perhaps, suggest that not enough time will go into examining the submissions. Afriyie, who is the vice-chair of the All-party Parliamentary Group for Vaping, suggested the U.K.’s civil servants were well informed and committed to the U.K.’s principle of harm reduction and would make a good job of reviewing the consultation, ensuring submissions were evidence based and making pragmatic recommendations to ministers. I wish I were so confident.
Civil servants presumably wrote the consultation, which is not in my view objective but comes with an agenda. The sixth paragraph kicks off with an almost meaningless statement: “No other consumer product kills up to two-thirds of its users.”
Glossing over the fact that no “product” had been identified by this stage of the text and that, therefore, to refer to “no other product” was meaningless, it is the case that “up to two-thirds” could mean none, which was perhaps not what the writers had in mind. And the word “kills,” though almost universally used, is clearly misleading. This is not to denigrate civil servants, just to point out that they are probably too few in numbers fully to perform the tasks assigned to them.

I worry the government believes that what it is dealing with here is not a health matter but a political one, especially given that a general election is likely to be called next year. Addressing the attendees about vaping by those underage, Afriyie, who is not standing at the next election, said, “… if any of your products, I mean any of them, have fancy colors or have packaging or have names or have flavors with names that would even vaguely appeal to young people, can I just say, just stop.
Because if next year I come to this conference and we’ve gone the route of Australia, do you know, it will be your fault. It will be your fault for not policing the industry and ensuring that you are absolutely responsible in your new role of healthcare.”
Of course, the UKVIA has no powers to police the industry. The association can encourage and cajole its members to ensure their products do not specifically appeal to those underage, but then it is unlikely that its members will be the ones to step out of line.
There is no doubt that vapes are getting into the hands of those who are too young legally to buy them, but, for a long time, the UKVIA has been telling the government about this trend and suggesting remedial actions that could be taken, including the licensing of retailers and heavy fines for those who recklessly sell vapes to those underage. Not only have such suggestions fallen on deaf ears, but successive Conservative governments have run a ruthless and reckless decade-long program of austerity that has, in part, decimated the organizations charged with policing retail activities.
According to a press note issued by Arcus Compliance on Nov. 9, the day before the forum was held, a leading academic at Imperial College London had reported that the budgets of one of those organizations, Trading Standards, had been halved: cut by an estimated £200 million ($254.04 million) since 2010.
Data gathered through Freedom of Information requests by Arcus Compliance reportedly showed that across 11 major provincial U.K. cities, which have a shared population of more than 5.5 million people, just 21 successful prosecutions had been made against retailers for underage/illicit sales between 2021 and early 2023. Further, the total amount of fines handed down across these cities over the same period was £2,188.
The managing director of Arcus Compliance, Robert Sidebottom, who was co-chair of the forum, said the concerning lack of enforcement in the form of prosecutions and penalties demonstrated the system was in serious distress.
“The government has now pledged £30 million to help intercept illegal tobacco and vaping goods at the border and to tackle youth access,” he was quoted in the press note as saying. “While this is a welcome development, we can’t just slap a multimillion-pound Band-Aid on the issue of underage and illicit vape sales and call it a day—especially if parliamentarians move on considerations to restrict the sale of disposable vapes.”
This was a theme addressed by John Dunne, the UKVIA’s director general, in his forum presentation. While acknowledging that it was necessary to urgently address what he described as the unintended consequences of sales to those underage, the sale of illicit products and the environmental damage caused by vapes, he pointed out that while current regulations were not being enforced, it made no sense to add further punitive regulations to the industry’s operations.

Dunne, whose job must have been made hugely more difficult in recent times by the almost constant churning within ministerial departments caused by the unstable, almost unhinged, turmoil within the ruling Conservative Party, expressed concern that the contents of the vaping consultation had been swayed by the court of public opinion, driven in turn by click-bait journalism, and therefore threatened “to undo all of the good work that our sector has done in being the most disruptive force in history in addressing the most preventable cause of death, which is smoking.”
The forum, which apparently is now the biggest business-to-business event in the U.K., was well organized and held within a comfortable, well-run venue, the QEII Centre in Westminster, London. There was a program of practical panel discussions and presentations, which included an address by Weinuo Ao, the secretary general of the China Electronic Chamber of Commerce, which, in cooperation with the UKVIA, organized the first trade delegation of Chinese companies to attend a UKVIA forum.
There was a panel session on the thorny, seemingly unresolvable, problem of trying to change public perceptions about vaping for the better and one on the equally thorny and divisive subject of addressing the environmental impact of vapes. It is almost painful to see how both of these issues, like the enforcement issue, are largely out of the industry’s hands.
Enforcement is in the hands of the government, and changing perceptions would require those currently spreading misleading information about vaping to stop what they are doing, while it is consumers who should, in their own interests, take the environmental issue in hand by not carelessly discarding their vapes.
One panel discussed the future of vaping, another looked at the future of retailing and yet another examined whether in the future it would be possible or even desirable to take harm reduction to a new level—perhaps beyond the 95 percent less risky figure normally quoted in the U.K.
Alongside the forum was a mini-exhibition, and an awards dinner followed, co-compared by Marina Murphy, senior director of scientific and medical affairs at ANDS, and Sairah Salim-Sartoni, founder of Salim-Sartoni Associates. Sixteen awards were up for grabs, including one for most supportive parliamentarian, which went to Afriyie.
Finally, whereas my take on the forum was that the most significant threats to the vaping industry in the U.K. were being caused by a lack of enforcement of current regulations, there was hope. For instance, from my observations, the government could take its enforcement activities to a new level simply by taking some lessons from Sidebottom and his co-chair, Jeannie Cameron, the CEO of JCIC International and the first woman to chair a UKVIA forum.
Sidebottom, who is ex-military, and Cameron ran the show like a military operation, and I don’t mean a retreat. Presentation timings were policed strictly with Sidebottom threatening stragglers with being grasped in a headlock and dragged from the stage and Cameron saying something about a whip. Unless I was mistaken, at least one participant was stopped mid-sentence …
















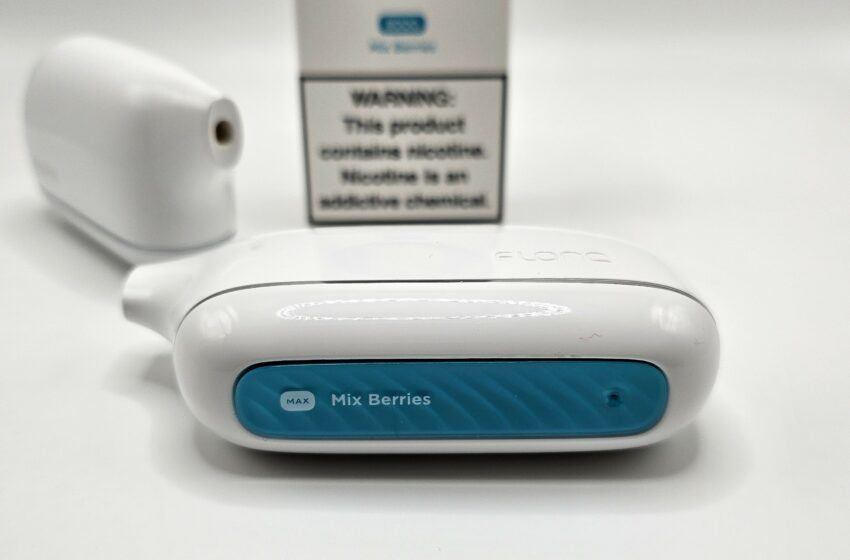
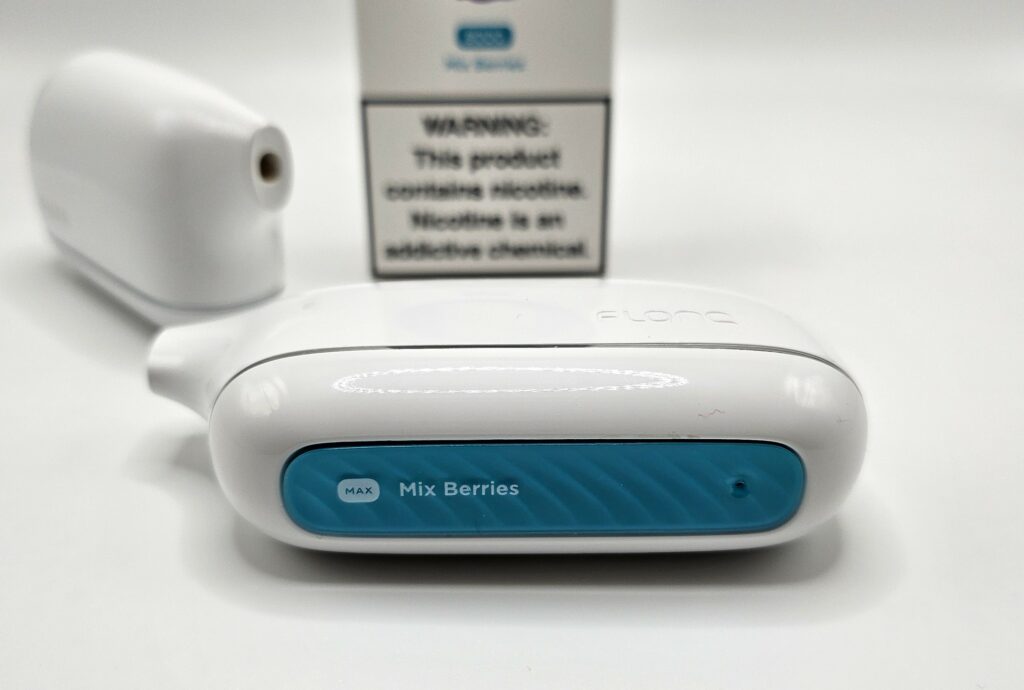
 Each Flonq Max comes included with a built-in mesh coil heating element. Mesh coils are ideal for pod systems as they require lower power to produce adequate vapor, which extends battery life and ensures peak efficiency when it comes to the e-liquid. The 14 mL capacity is advertised as the equivalent of 8,000 puffs, but this is difficult to determine without an included puff counter. However, as a rough guideline, the average user can expect large-capacity devices such as the Flonq Max to last for one week before the e-liquid starts to run dry.
Each Flonq Max comes included with a built-in mesh coil heating element. Mesh coils are ideal for pod systems as they require lower power to produce adequate vapor, which extends battery life and ensures peak efficiency when it comes to the e-liquid. The 14 mL capacity is advertised as the equivalent of 8,000 puffs, but this is difficult to determine without an included puff counter. However, as a rough guideline, the average user can expect large-capacity devices such as the Flonq Max to last for one week before the e-liquid starts to run dry.




