A new report published by the U.K. public health agency Knowledge Action Change (KAC) demonstrates an urgent need to scale up tobacco harm reduction, which enables smokers to switch to safer nicotine products, eliminating the smoke that causes death and disease.
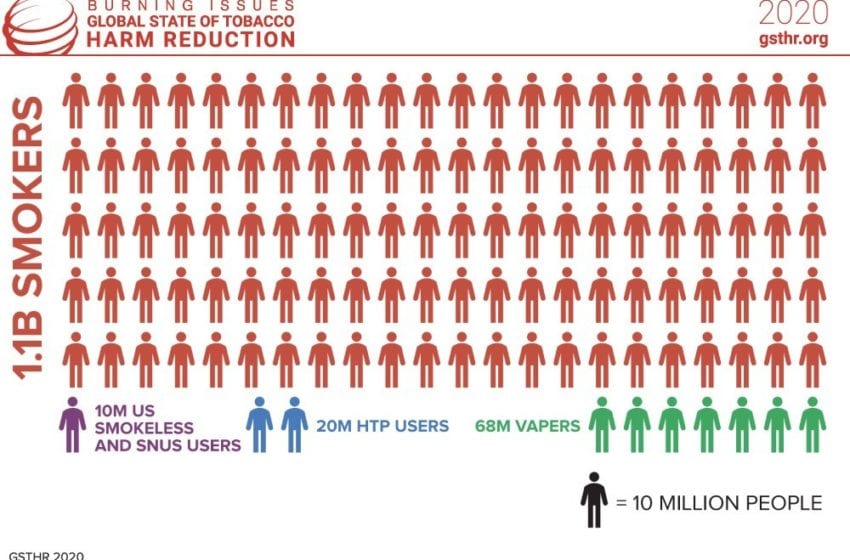
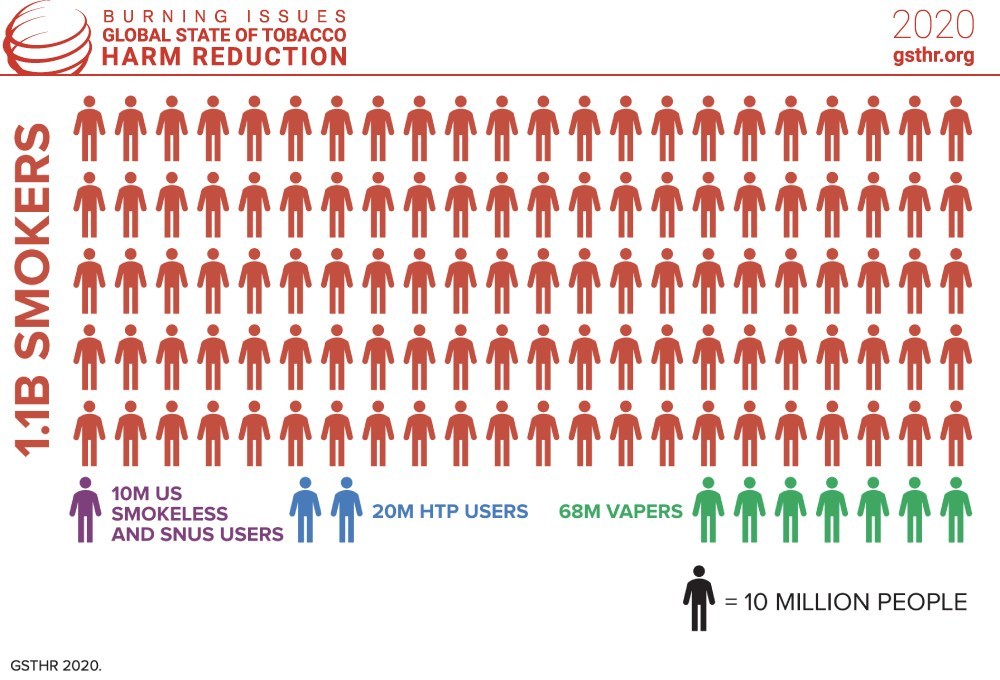
Titled “Burning Issues: The Global State of Tobacco Harm Reduction (GSTHR),” the report reveals that an estimated 98 million people use these products globally, including 68 million vapers, 20 million users of heated tobacco products and 10 million consumers of U.S. smokeless or pasteurized oral snus.
While showing huge demand for safer alternatives, these numbers are dwarfed by the global total of 1.1 billion smokers—a figure that has remained static for two decades despite billions spent on tobacco control. Eight million people die due to smoking-related disease every year.
During the report’s online launch, co-hosted with Lilongwe-based NGO THR Malawi on Nov. 4, the report authors showed that both access to and adoption of safer nicotine products largely remains the preserve of higher income countries, while 80 percent of the world’s smokers live in low- and middle-income countries poorly equipped to implement tobacco control or treat smoking-related disease.
The report further shows how tobacco control policy at the World Health Organization is being influenced by billions of dollars from U.S. foundations campaigning against tobacco harm reduction, while misinformation is discouraging smokers from switching to safer products.
KAC Director Gerry Stimson believes the world’s 1.1 billion smokers deserve better. “Integrated into tobacco control, harm reduction could be a gamechanger in the battle against noncommunicable disease,” he said in a statement. “Global tobacco control policymakers must listen to consumers and deliver policies that genuinely focus on reducing smoking-related deaths by all available means.”
Professor David Nutt argued that to reject the opportunity of tobacco harm reduction “is perhaps the worst example of scientific denial since the Catholic Church banned the works of Copernicus in 1616.”