The Center for Black Equity (CBE) called on the U.S. Food and Drug Administration and the Center for Tobacco Products to grant broad approval to a full range of nicotine e-cigarettes, also known as vapes, in a major step toward closing the significant harm reduction and health equity gaps perpetuated by current FDA tobacco policies, according to a press release. The approval of vaping products would benefit Black and LGBTQ+ populations disproportionately impacted by the negative health effects of smoking, including cancer, according to the CBE.
The CBE’s call for expansive regulatory approval of e-cigarettes, including flavored vaping products, comes as the CBE released an econometric report that, for the first time, quantifies the benefits of switching from smoking to vaping in terms of lives saved, GDP benefit and healthcare savings.
The report was authored by Robert J. Shapiro, former undersecretary of commerce for economic affairs and advisor to former President Clinton, former President Obama and President Biden. Shapiro’s report found that between 2010 and 2022, shifting from smoking to vaping saved 113,000 lives, preserved $137 billion in GDP and saved $39 billion in healthcare costs—and that the availability of e-cigarettes reduced the number of smokers in the U.S. by 6.1 million during that same period.
“Championing meaningful harm reduction initiatives for Black and LGBTQ+ communities has been an elusive but essential aspect of effective public health advocacy for decades,” said Earl Fowlkes, president and CEO of the CBE. “If the Biden administration and the FDA are serious about health equity and harm reduction, especially when it comes to the president’s Cancer Moonshot initiative, the science is clear: Broad approval of flavored vaping products will save Black and LGBTQ+ lives, reduce smoking and drive meaningful progress in lowering preventable cancer rates in the U.S., especially among the most vulnerable populations.”
The report also reviewed existing academic and medical literature on vaping versus smoking to examine and verify the substantial scientific evidence that e-cigarettes have a drastically lower risk profile than cigarettes and can help individuals successfully reduce smoking or quit altogether.
“The single most effective way to help people stop smoking, which kills 480,000 people per year, is to encourage them to switch to vaping, which kills no people per year,” said Shapiro.
“The Center for Tobacco Products needs to be honest with American smokers—especially those in Black and LGBTQ+ communities who smoke at disproportionately higher rates—and proactively convey the substantial health benefits of shifting from smoking to vaping,” Shapiro said. “Future FDA policy on tobacco and nicotine products should draw on the well-established scientific evidence regarding the relative risks of e-cigarettes versus cigarettes and the utility of people using vaping to stop or reduce their smoking.”
The report also squarely examined the primary concern of critics of e-cigarettes, the supposed “youth vaping epidemic,” to which formal FDA approval of vaping products would allegedly contribute. “The supposed ‘youth vaping crisis’ narrative that has existed for some time in the media and, curiously, in public health conversations at the FDA is unfounded,” Shapiro continued. “The U.S. Centers for Disease Control and Prevention’s own data show that adolescent vaping has declined substantially in recent years—receding to 2014 levels, well below the 2019 peak—and that most young people who vape do so on an irregular or occasional basis without becoming dependent on nicotine.”
“The FDA and the Center for Tobacco Products have an obligation to follow the science, support harm reduction and health equity, and advance—rather than stall—President Biden’s Cancer Moonshot,” concluded Fowlkes. “The FDA must acknowledge the evidence-based benefits of switching from smoking to vaping and aggressively educate Black, LGBTQ+ and other smokers about those benefits. Failure to approve a wide range of vaping products is an abdication by the FDA of its public health responsibility to Black and LGBTQ+ individuals across the country who desperately want to access a way to quit smoking that actually works.”












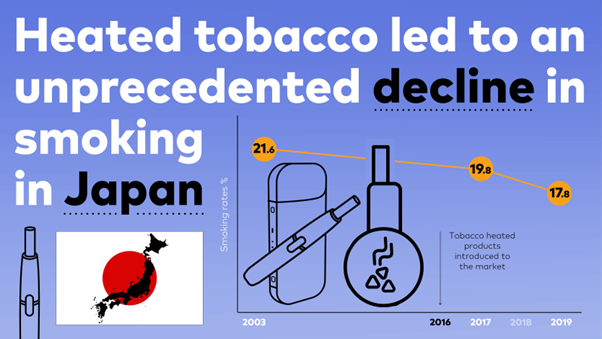
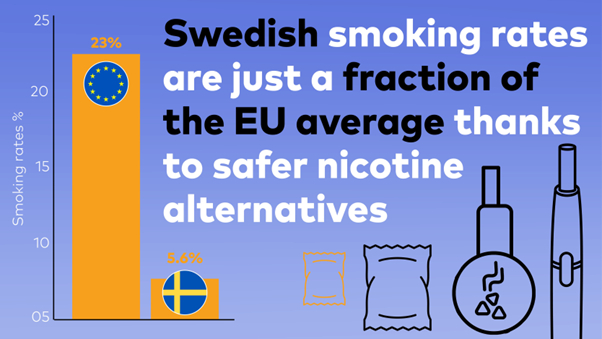
 The Tholos Foundation, in partnership with Japan-based Pacific Alliance Institute and Sweden-based consulting firm Scantech Strategy Advisors, has released a policy paper,
The Tholos Foundation, in partnership with Japan-based Pacific Alliance Institute and Sweden-based consulting firm Scantech Strategy Advisors, has released a policy paper,