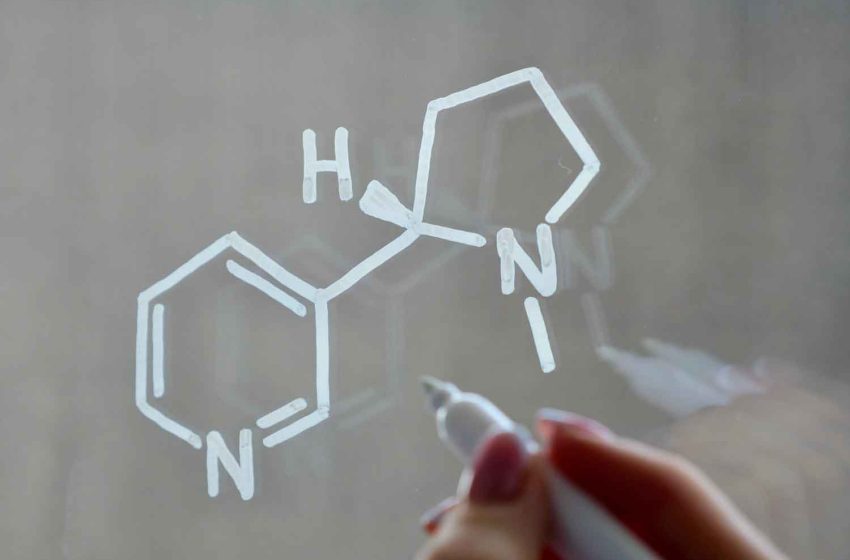
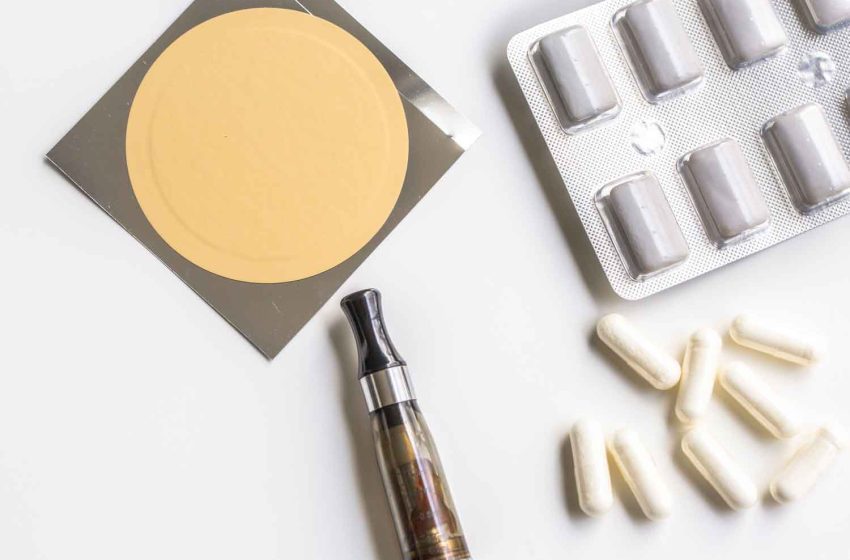
Northerner, an online seller of smokeless tobacco and nicotine products for 25 years, said starting this month, it will exclusively sell tobacco-free nicotine pouches and other modern oral nicotine products.
The announcement marks a new chapter in the company’s commitment to public health and the tobacco harm reduction movement, the Missouri City, Texas-based company said.
“Having been in the smokeless tobacco business for 25 years, the decision to move away from tobacco has not been easy,” said Sarah Krysalka, senior director of commercial partnerships and external affairs at Northerner. “But the trends are clear.
“More Americans are choosing tobacco leaf-free options, and we aim to stay at the forefront of this movement by offering the best assortment of modern oral nicotine products to meet the evolving needs of adult consumers.”
With the rise of non-combustible nicotine options like pouches, the company said it recognizes the need to adapt to the shifting market landscape and provide consumers with tobacco leaf-free and other modern oral nicotine product alternatives to combustible cigarettes, according to a press release.
“By leaving the tobacco market, Northerner will shift its focus to fulfilling the growing demand for nicotine pouches and other modern oral nicotine products,” said Krysalka. “Our goal is to cater to the changing needs of adult nicotine users while educating tobacco consumers about tobacco leaf-free nicotine alternatives.”
Northerner.com is a website operated by Northerner Scandinavia Inc, a U.S. subsidiary of Northerner Scandinavia AB and Haypp Group.