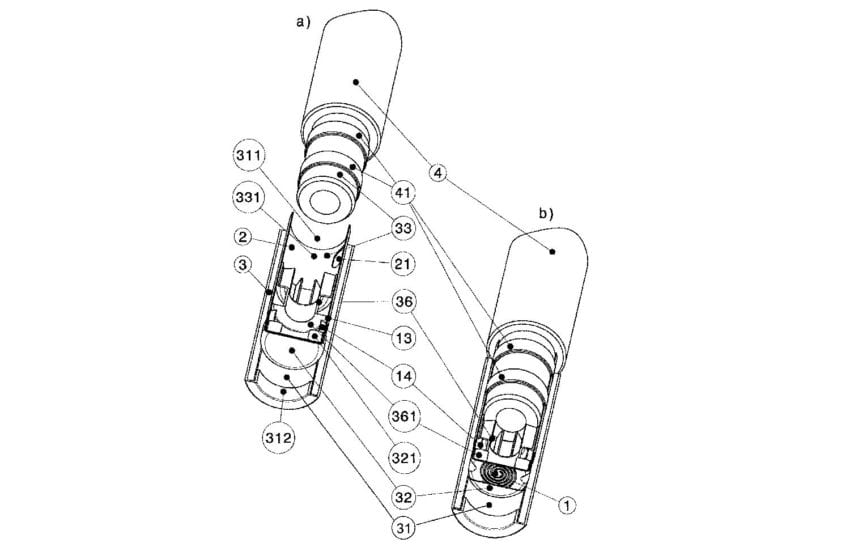
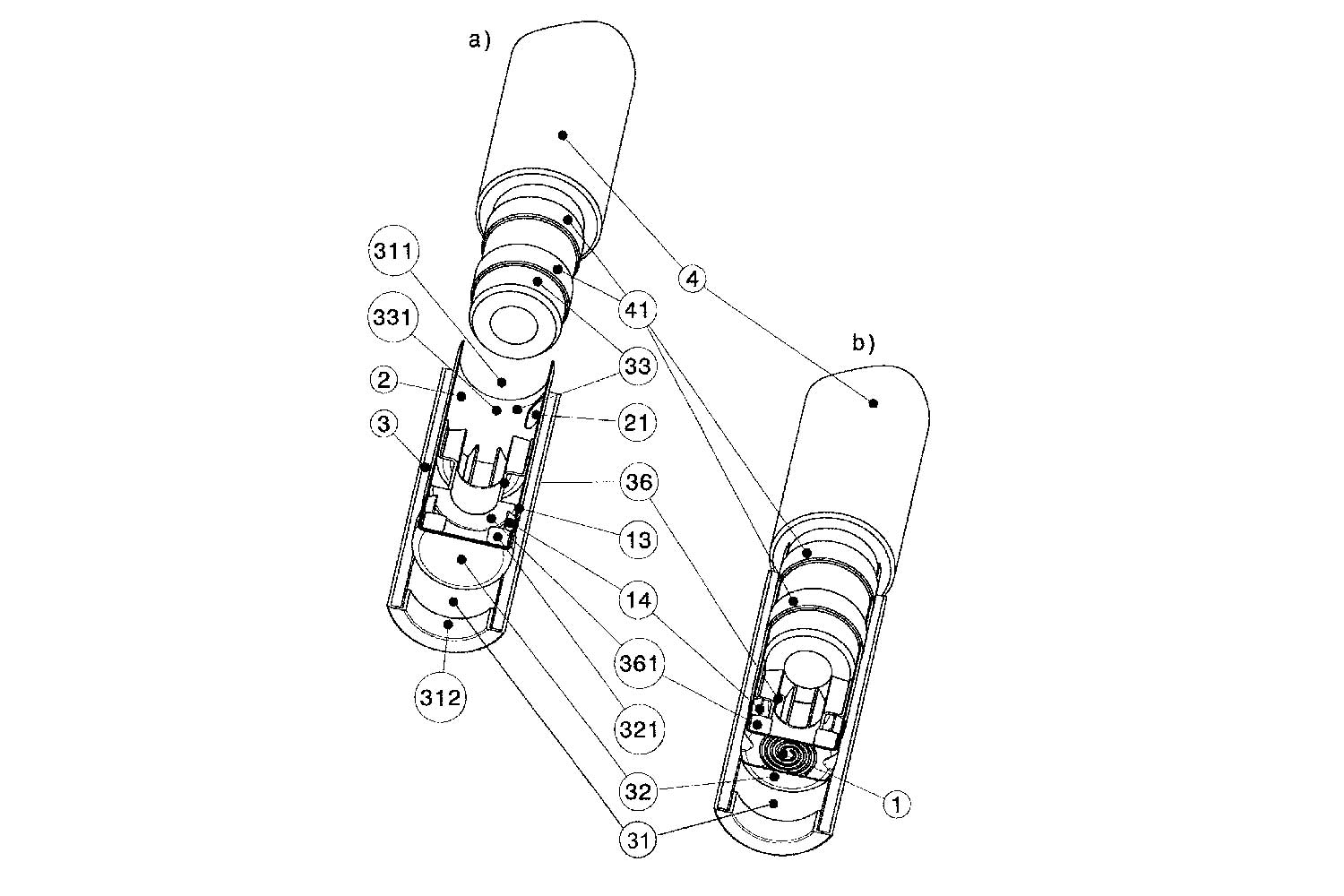
Kaival Brands Innovations Group has been granted two copyright protections and two patents by China.
The first patent, China Patent No. 202020067263.5, is a utility model patent, and relates to the nozzle components of the Bidi Stick. The nozzle components play an integral role in delivering a consistent user experience. The second patent, China Patent No. 202030052391.8, is a design patent that covers the entire Bidi Stick product. Bidi Vapor has also secured copyrights for both the Bidi Stick and Bidi Cares names.
Kaival believes that the Chinese vapor market presents a considerable business opportunity. Statista data projects the China combustible cigarette market to top $220 billion in 2021. Vape products are quickly gaining market share in China and if a mere 10 percent of combustible cigarette smokers transition to vape, China would be a $22 billion vape market opportunity. By comparison, Grandview Research anticipates the U.S. vape market to reach $7.4 billion in 2021.
“The copyright and patent protection representations received from China are the first step in our planned journey to introducing the Bidi Stick into one of the world’s largest markets for vape products, China,” said George Chuang, independent director of Kaival Brands, in a statement. “I look forward to advising the company in my role as a board member in interfacing with potential distribution partners in China.”
“Receiving two patents from China, along with copyright protections, should enhance our efforts to more effectively eliminate counterfeit players from the market, and being afforded these protections within a difficult market further validates our best-in-class product lineup” says Niraj Patel, founder and chief executive officer of Kaival Brands and Bidi Vapor. “Both Bidi Vapor and Kaival Brands are adamant about exceeding compliance standards in every global market, and as such our products are intended exclusively for adults 21 and over.”
Following the latest patents, Kaival has intellectual property protections in the United States, the European Union, Australia and China. “We believe this puts us in a strong position to pursue new global markets that we have already received regulatory approval to enter,” says Patel.