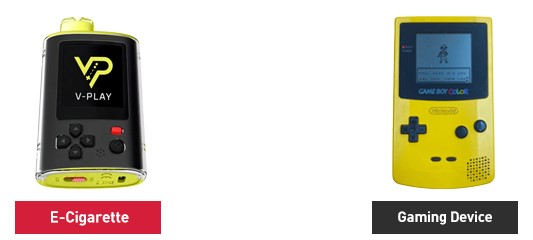
The U.S. Food and Drug Administration issued warning letters to eight online retailers and one manufacturer for selling and/or distributing unauthorized flavored, disposable e-cigarettes.
Some of the unauthorized products cited in the warning letters are marketed under brand names for disposable products, including Geek Bar and Lost Mary, according to the FDA. Other unauthorized products cited feature the names and/or images of celebrities.
The firms receiving these warning letters sold and/or distributed e-cigarettes in the United States that lack authorization from FDA to be legally marketed in the U.S., which is in violation of the Federal Food, Drug, and Cosmetic Act.
In addition to the violations mentioned in the warning letters, the firms were warned to address any violations that are the same as, or similar to, those stated in the warning letter and to promptly take necessary actions to comply with the law.
Failure to promptly correct the violations can result in additional actions such as an injunction, seizure, and/or civil money penalty.