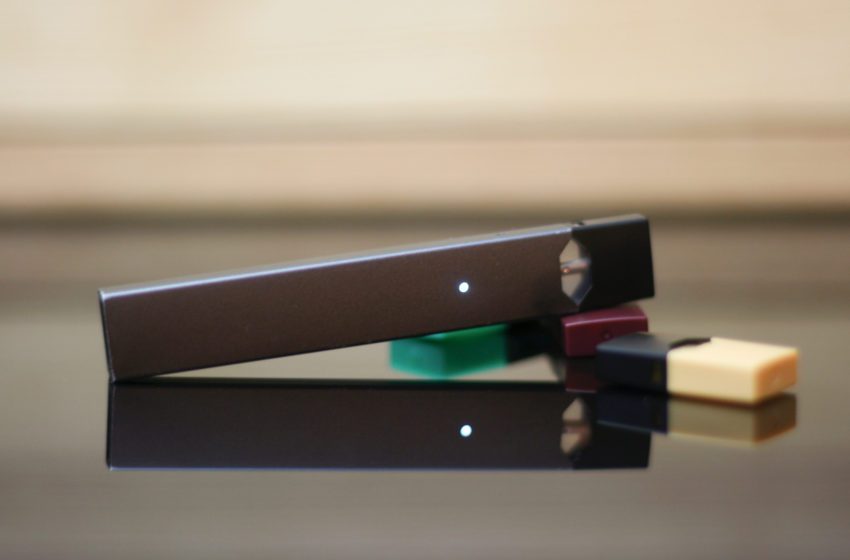
Today, the U.S. Food and Drug Administration confirmed what many had already been anticipating: Juul Labs must remove all currently marketed Juul products from the U.S. market.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” said FDA Commissioner Robert M. Califf. “The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market. We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
These marketing denial orders (MDO) pertain only to the commercial distribution, importation and retail sales of these products, and do not restrict individual consumer possession or use—the FDA cannot and will not enforce against individual consumer possession or use of Juul products or any other tobacco products.
“We respectfully disagree with the FDA’s findings … intend to seek a stay and are exploring all of our options under the FDA’s regulations and the law, including appealing the decision and engaging with our regulator,” said Joe Murillo, chief regulatory officer at Juul Labs, said in a statement. “We remain committed to doing all in our power to continue serving the millions of American adult smokers who have successfully used our products to transition away from combustible cigarettes, which remain available on market shelves nationwide.”
After reviewing the company’s premarket tobacco product applications, the FDA determined that the applications lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.
In particular, some of the company’s study findings raised concerns due to insufficient and conflicting data—including regarding genotoxicity and potentially harmful chemicals leaching from the company’s proprietary e-liquid pods—that have not been adequately addressed and precluded the FDA from completing a full toxicological risk assessment of the products named in the company’s applications.
Matthew L. Myers, president of the Campaign for Tobacco-Free Kids, called the FDA decision “the most significant action the FDA has taken to reverse the youth e-cigarette epidemic. Juul, more than any other product or company, has been responsible for creating and fueling the youth e-cigarette epidemic.”
The FDA says that, to date, it has not received clinical information to suggest an immediate hazard associated with the use of the Juul device or Juul pods. However, the MDOs issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the Juul products.
“There is also no way to know the potential harms from using other authorized or unauthorized third-party e-liquid pods with the Juul device or using Juul pods with a non-Juul device,” the agency wrote in a statement.
“The FDA is tasked with ensuring that tobacco products sold in this country meet the standard set by the law, but the responsibility to demonstrate that a product meets those standards ultimately falls on the shoulders of the company,” said Michele Mital, acting director of the FDA’s Center for Tobacco Products. “As with all manufacturers, Juul had the opportunity to provide evidence demonstrating that the marketing of their products meets these standards. However, the company did not provide that evidence and instead left us with significant questions. Without the data needed to determine relevant health risks, the FDA is issuing these marketing denial orders.”
Gregory Conley, president of the American Vaping Association, asserted on Twitter that the Juul decisions were “manufactured” and “complete nonsense.”
Andrew Bagley is the owner of the Illuminati Smoke Shop in Columbia, South Carolina, and says even with the popularity of Juul, he doesn’t have concerns about the ban’s effect on sales, reports News19.
“I’m not concerned,” he said. “We have quite a few other vape products that are PMTA approved, which just means at this point we are legally allowed to sell them and Juuls were a very small percent of our sales, it’s not that big of a deal.”