
The U.S. Food and Drug Administration issued warning letters to nine online retailers and one manufacturer for selling or distributing unauthorized disposable e-cigarettes designed to resemble smart technology, including smartphones and gaming devices.
The products mentioned in the warning letters are promoted as having various designs and functions that might attract young people, according to an agency press release. These include features like playing games, connecting to smartphones, receiving text or call notifications, playing music, and customizing products with personalized wallpaper.
“These products may resemble smart devices, but there’s nothing smart about them,” said Brian King, director of FDA’s Center for Tobacco Products. “They’re illegal to sell and a flagrant attempt to target kids.”
The agency states that the designs of the unauthorized products cited in the warning letters are likely to appeal to youth because they help conceal the nature of the products as tobacco products from parents, teachers, or other adults. Example images of unauthorized products cited in the warning letters compared to electronic devices on the consumer market, such as smartphones and gaming devices.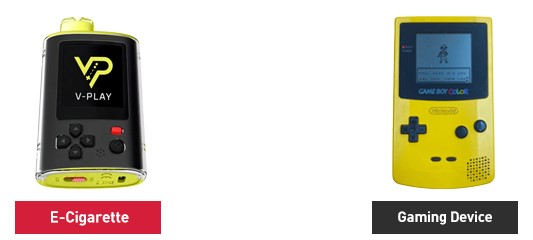
“The firms receiving these warning letters sold and/or distributed e-cigarettes in the United States that lack authorization from FDA to legally market a new product, which is in violation of the Federal Food, Drug, and Cosmetic Act,” the release states.
In addition to the violations mentioned in the warning letters, the retailers and manufacturer were warned to address any violations that are the same as, or similar to, those stated in the warning letter and promptly take any necessary actions to comply with the law. Failure to promptly correct the violations can result in additional FDA actions such as an injunction, seizure, and/or civil money penalty.
“FDA is steadfast in our commitment to enforce the law,” said John Verbeten, director of CTP’s Office of Compliance and Enforcement. “We will continue to take appropriate measures, working hand in hand with our federal enforcement partners, to address unauthorized tobacco products, especially those most appealing to youth.”



















