FDA Warns Companies For Selling ‘Smart’ Vapes
The products in the warning letters have designs and functions that may attract young people
Thousands of manufacturers and importers submitted premarket tobacco product applications (PMTAs) to the Food and Drug Administration by the Sept. 9, 2020, deadline to continue selling their products in the United States. How have these applications fared? Who will remain in the market and who willl exit? Keep up with the latest developments in market authorizations in this dedicated section of our website.


The products in the warning letters have designs and functions that may attract young people


SCOTUS announced Monday, Dec. 2, 2024 as the date for the FDA v. Triton Distribution hearing.


No matter which way the justices rule, they are not expected to address the merits of the FDA’s denial.


Qnovia will now initiate a trial to determine pharmacokinetics, safety and tolerability self-administration.


After July 1, 2025, those caught vaping will risk a fine $115 and sellers of e-cigarettes will be subject of a penalty.
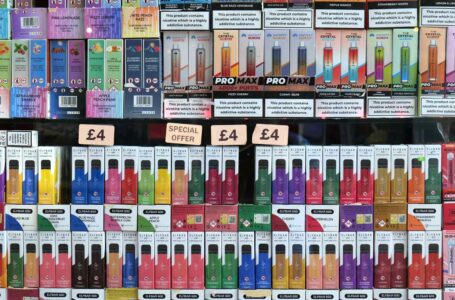

Flavors like fruit, candy, mint and desserts accounted for more than 80 percent of sales.


CoEhar researchers assessed respiratory symptoms among adults who had never been habitual smokers but vaped.


The suit accuses Swedish Match of violating antitrust laws concerning the market for nicotine pouches.


The hospital brought the case to the attention of the Buri Ram provincial public health office.



House Bill 11, which dealt with youth vaping, doesn’t have consequences for offenders.


Disparities in tobacco use persist by race and ethnicity, income, education, and other factors, the report states.


Net revenue from tobacco and “next-generation” products rose 4.6 percent in the year ended September 30.


Anyone selling disposable vapes from June 1, 2025 could get a £200 fixed-penalty notice.


The technology aims to prevent youth access while expanding adult market access to flavored products.

CAPHRA cites evidence from Japan demonstrating the health benefits of embracing safer alternatives.


THR activists call for regulation rather than prohibition to drive down the health toll of tobacco use.


At the recent GTNF in Athens, stakeholders debated how to responsibly advance innovation.


Regulators often run on a campaign of hypocrisy when confronting vaping products..


The RespiRx, the first inhalable nicotine-replacement therapy, gains IND clearance.


Industry experts explain why vaping regulations vary throughout Southeast Asia.


The 2024 InterTabac and InterSupply trade shows continue to go to the next level.


Finding solutions to hazardous waste from vaping products is a growing concern.


Click on any country to view its vapor industry news


Once individuals switch from tobacco smoking to vapes, there is no clear baseline for before and after use comparison,


The rate of ‘battery thermal runaway incidents’ on aircraft hit a record high last year, according to a new study.


Its proposal targets substances required to create popular vape flavors such as caramel, raspberry and vanilla.


On Aug. 18, Vaporesso will celebrate with a music party featuring extreme sports in Nice, France.



House Bill 11, which dealt with youth vaping, doesn’t have consequences for offenders.


The survey showed retailers would support checks coming into force where the minimum age to purchase a vape.


The UKVIA supports the introduction of vape licensing to fund a nationwide enforcement program.


Sellers of age-restricted products must stay abreast of rapidly changing laws and regulations, says We Card.


Kennedy’s position on vaping, nicotine, and tobacco harm reduction (THR) remains unclear.


The modified risk granted orders issued by FDA are specific to the Swedish Match and expire Nov. 7, 2032.
The products in the warning letters have designs and functions that may attract young people


The e-cigarettes, which contain etomidate, have rapidly gained popularity among Taiwanese vapers.
Presentations highlighted new opportunities to accelerate harm reduction, including those involving genomics and artificial intelligence.
The justices took up the FDA’s appeal filed after a lower court ruled against the regulatory agency.
The statute will also allow a caregiver to assist a qualified patient with use, possession and acquisition.
The justices took up the FDA’s appeal filed after a lower court ruled against the regulatory agency.
Where you buy your e-cigarettes may determine if you will be a successful quitter.
Scientists urge US to screen for COVID-19 in vaping-related lung disease EVALI and share data discovered.
The Department of Education expressed its full support towards stricter measures on ENDS products.