R.J. Reynolds Vapor Co. (RJRV) has petitioned the U.S. Patent and Trademark Office for a review of six claims relating to the basic functionality of e-cigarettes in a patent assigned to Philip Morris Products, reports Law Street Media.
RJRV argues that the patent describes an approach that dates from 1990 and has “become accepted in view of its comparatively easy technical realizability in combination with its convincing functionality.’”
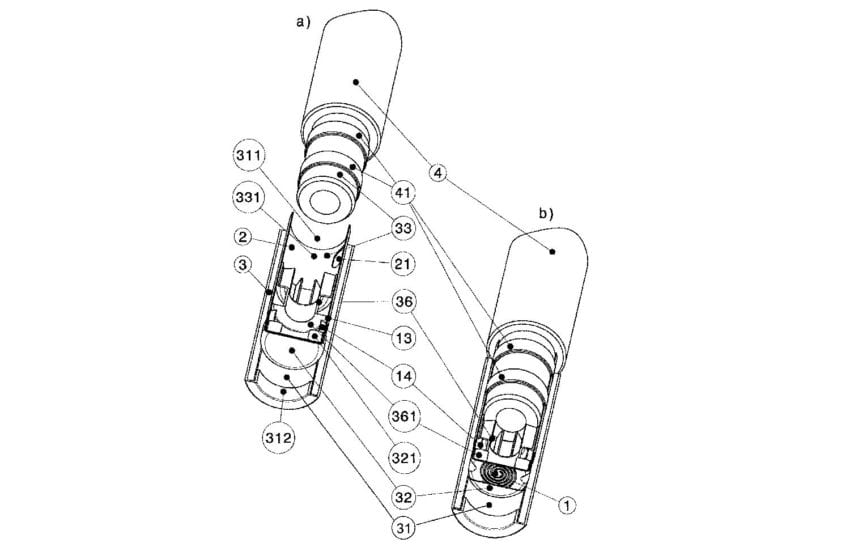
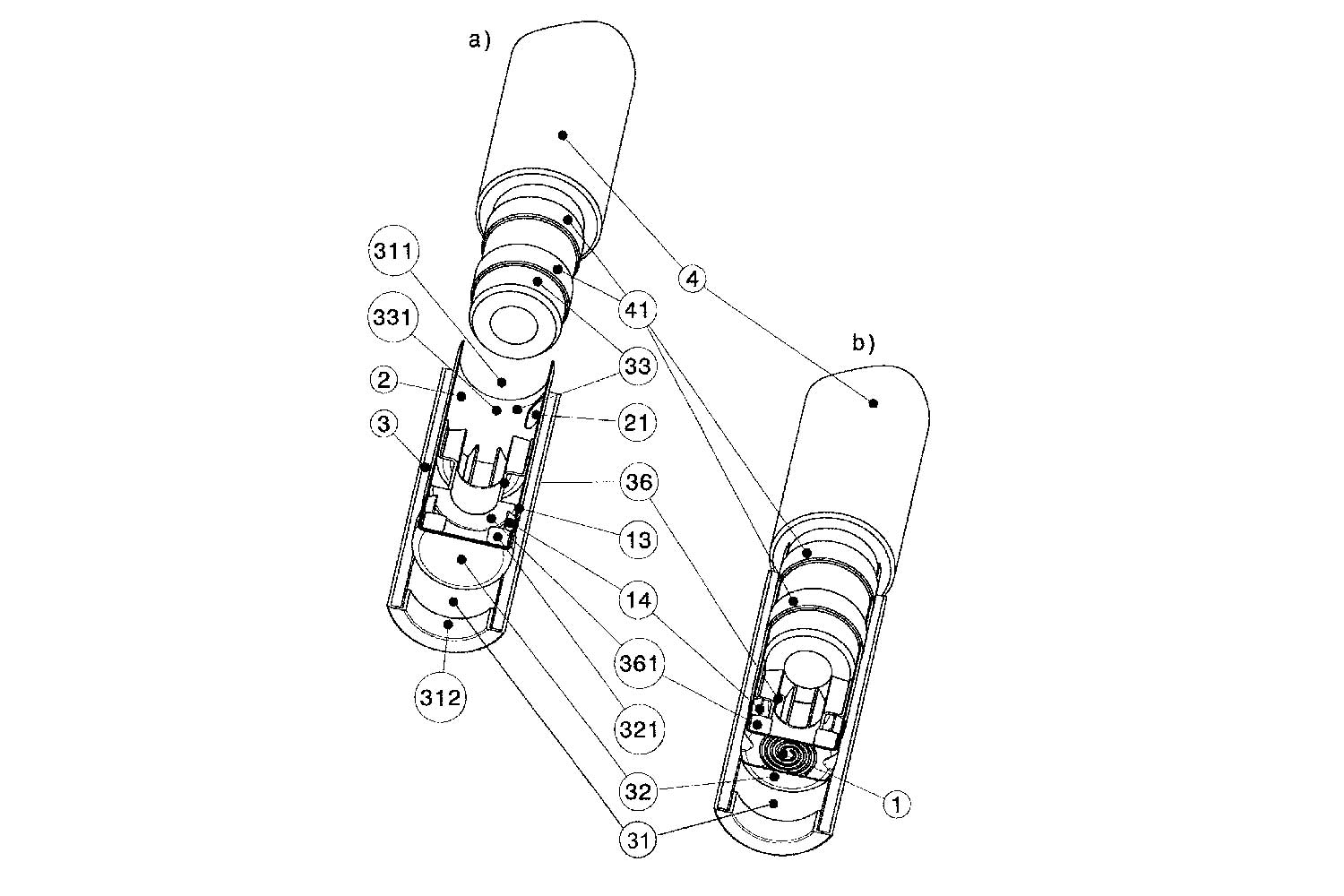
According to the filing, there are disadvantages in the prior technology that the asserted patent claims to fix, such as the increasing contamination of the vaporizing unit throughout its life, a fluid leak, and that due to its design, the e-cigarette’s length cannot be shortened.
RJRV takes issue with the patent’s six claims on the basis that to a person having ordinary skill in the field, it would have been obvious to combine previous inventions to overcome the claimed deficiencies.
RJRV requests the cancellation of the claims as unpatentable.
It’s not the first time that Reynolds and Philip Morris have quarreled about intellectual property. In June 2020, Philip Morris International filed counterclaims against Reynolds for patent infringement in the federal court action that RJR commenced against PMI and Altria, PMI’s IQOS distributor in the U.S., on April 9, 2020 in the Eastern District of Virginia.