
In less than two years, Canada’s Vaping Industry Trade Association has made great strides in saving lives.
By Timothy S. Donahue
Like nearly everywhere else in the world, the vaping industry in Canada is under fire. Lawmakers at all levels of government are seeking to or already have raised taxes, criminalized public vaping and banned flavored e-liquids. Moving into 2021, it is expected that the Canadian government will continue to try to ban flavors or possibly make regulations so onerous they kill the industry.
These kinds of regulatory actions have the potential to push former smokers back to combustible cigarettes, leading to an increase in the number of deaths caused by smoking every year. This is a major concern for trade industry groups like Canada’s Vaping Industry Trade Association (VITA). While relatively new to the game, VITA has quickly grown into one of the largest and most vocal vaping trade associations in Canada.
Daniel David, a former smoker who used vapor products to quit traditional cigarettes, says that in mid-2019, VITA opened its doors with a goal of keeping Canada’s vaping industry from being regulated into oblivion. David, who opened Canada’s first vape shop in 2010, said VITA was founded after the Canadian government released its rules for electronic nicotine-delivery systems in mid-2018 (Tobacco and Vaping Products Act). He now serves as the advocacy group’s president and CEO.

When David began his journey in the vaping industry, there were not any regulations on vaping in Canada. He says the industry just stepped up and figured out regulations themselves. They implemented age restrictions and standards for testing e-liquids. He says he got into advocacy because, as a retailer, when people would come into his shop and discover vaping products, they would often cry and give him a hug. They would tell him that they had resigned themselves to dying from smoking cigarettes. “We got them vaping, and they didn’t want to go back to smoking,” he said. “It’s that kind of experience that makes vapor advocates so passionate because we know what the potential is with this product. The science is there.”
When he and his team decided to start VITA, David made the decision to dedicate himself to the organization full time. He asked his wife to take over running the business. “I made the decision that this is really important,” he said. “I needed to be able to dedicate my full time and attention to advocacy. I got into it early on because I wanted this industry to become something real, a legitimate industry. And I wanted to make sure that the products that we are selling are as safe and effective as they possibly can be. And when we started this adventure, there was a regulatory gap.”
When regulations came, they arrived quickly. They were also all over the board. Some provinces were implementing age restrictions (which VITA supports), while others were taking a more hardcore approach and wanted to ban flavors (which VITA does not support). Then a lung disease that many health groups and media outlets blamed on nicotine vaping began to envelop the industry.
Troubled waters
VITA got its start during the peak of the e-cigarette or vaping product use-associated lung injury (EVALI) crisis. The term was coined by the U.S. Centers for Disease Control and Prevention (CDC) for a dangerous, newly identified lung disease that was eventually linked to vaping black market marijuana products. However, the CDC had already falsely stated that nicotine vaping may be a contributor, and the global media pounced. By the time the CDC admitted that no nicotine vaping products had been a source of EVALI, the damage had been done. Then came the youth vaping crisis. Then the Covid-19 pandemic. It was the perfect storm for anti-vaping groups to pursue their agenda.

“With all of the threats, public perception [for vaping products] took really big hit. EVALI, Covid[-19], youth use … it takes up the media cycle now. So, it’s a challenge in one respect to get mainstream media to say anything that’s factual, that’s relevant about vaping. Mind you, the media wasn’t ever really saying anything too positive about vaping prior,” David explains. “That hasn’t changed. The point is that these threats that we’re facing as an industry moving forward are, by far, the biggest threats that our industry has ever faced. We had multiple provinces that were coming out with new regulatory regimes around vaping, and it was all due to EVALI. A lot of the members in the industry started to see what was happening and it became vitally important that we all work together, or the industry would die.”
There are six founding members of VITA—Dvine Laboratories, Juul Labs Canada, Valor Distributions, Atelier de Saveurs LaVapeShop, Imperial Tobacco Canada (Vuse) and JTI Canada Tech (Logic). The companies committed to contributing $100,000 each year for three years, which provided a seat on the board for three years. This multi-year financial commitment allows VITA to hire staff and retain legal, government relations and public relations support. The investment also provided capital for programs and initiatives, such Covid-19 protocols and preventing youth access.
Today, VITA has 44 members and is governed by a 12-member board that represents multiple segments of the vaping industry from small manufacturers, distributors, suppliers and vape shops to the large multi-national tobacco companies that are also moving into the vaping industry. Traditional tobacco companies like Juul Labs and Logic have the same voice on the board as nontobacco affiliated companies such as Dvine Laboratories and LaVapeShop. The six founders also have an (equal) weighted vote share of 40 percent and the six elected (nontobacco affiliated) companies have a weighted vote share of 60 percent.
“We’ve put ourselves into a position where we have a lot of intelligence; we have a lot of highly experienced professional people that are part of this group. We have resources that we haven’t really had in the past,” said David. “There is still an aspect [of this industry] that people are hesitant about. There’s still a lot of conspiracy theories out there, especially under the view that the tobacco-affiliated [vape] companies just want to see the industry die so they can continue to sell cigarettes. And that’s just not true. We are dedicated to harm reduction. We want to serve as a voice for the entire category, regardless as to the distribution channel.”
Closer to home
The Canadian vapor industry is complicated. Restrictions vary across the country. Youth use is a major driver of restrictions in Canada. David says that VITA supports age restrictions, and “minors should not use or have access” to vaping products. “Vaping products are for current adult smokers only, not youth or nonsmokers,” he says emphatically. “Governments should focus on the issues of access, enforcement and education.”
Restrictions dealing with the Covid-19 pandemic were especially burdensome. In Manitoba, for example, the government established a list of essential businesses and did not include vape shops. The province then forced all nonessential businesses to close. They even went a step further and only deemed certain products as essential. This meant that convenience stores were open as an essential business; however, the stores could only sell essential products, according to David.
“They have a list of what is essential and what is not. On that list, you have tobacco products are essential, and it specifically states vape products are not. So, the smoking population and vaping population in Manitoba [are] faced with a choice,” explains David. “They can’t get their vape products from vape shops because they’re all closed. If they were to go into a gas station or convenience store, which are open, they are only allowed to buy tobacco cigarettes. The vape products that are two inches below that tobacco cigarette, because it’s nonessential, customers can’t purchase.”
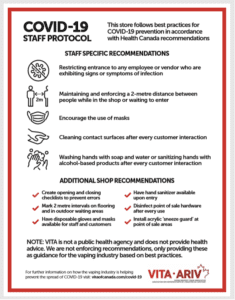
In April 2020, Nova Scotia instituted a 20 mg/mL nicotine cap coupled with a flavor ban. Neilson data showed that cigarette sales increased over 25 percent—a rate four times higher than surrounding provinces, according to David. Moreover, 50 percent of all specialty vape shops immediately closed. Hundreds of Canadians were out of work.
Then, in July 2020, the Canadian government unveiled new regulations related to the marketing of vaping products. The new rules prohibit the display of any branding in a “place or manner visible to young people and will ensure that vaping devices are not visible at point of sale in places where young people might be present.”
By December 2020, Nova Scotia’s regulatory framework had creeped its way across the country and into Canada’s House of Parliament. The federal government proposed lowering allowable nicotine levels in regulated vapor products to 20 mg/mL, which David said would minimize their value to adult smokers seeking to transition to less risky products. “Considering the disparity of harm between vaping and smoking, we don’t understand why the federal government would be using Health Canada resources during a global pandemic to explore making it harder for adult smokers to switch to a reduced risk product.”
Canada continues to pursue several vapor industry regulations. It has already passed multiple measures, including implementing child-resistant container (CRC) usage nationwide. VITA applauded the effort for e-liquid bottles but was concerned about the challenges and timeframe of applying the same standard to tank/pod hardware. However, the VITA team has been actively engaged with international device manufacturers, associated companies and Health Canada regarding the implementation of the new requirements for refillable tanks and pods. In support of compliance efforts, VITA even developed a list of certified products that are currently (or soon to be) available for retailers on the Canadian market.
“The CRC list has been set up so that it can be updated live, meaning that as new products are certified and released, the list will be updated and automatically distributed to subscribers at the end of the week,” says David. “Stakeholders are encouraged to share this within the industry and to notify VITA staff of any certified device currently not on this list.”
The good fight
David says there is not time to rest. In order to continue to combat the misconceptions surrounding the vapor industry, VITA had to start at the ground level. The organization launched several programs for member retailers. Age verification, recognizing fake IDs, best practices and business law are just some of the programs offered, according to David. VITA also breaks down the regulatory and legislative responsibilities of different industry segments by province because the rules change throughout the country.
“When the pandemic hit, VITA developed Covid-19 prevention measures, including specific guidance and signage for members. When the industry is facing something like we’re facing now … Covid-19 had a really big impact because we had to make a major switch,” said David. “We also conducted several webinars for Covid[-19], such as financial support programs from the government. We brought somebody in to talk about what was available and then outline how you would apply.”
VITA also designed and created a series of webinars for e-liquid manufacturers that were considering retooling to produce hand sanitizer. David said that, like many countries, Canada ran out of hand sanitizer early in the Covid-19 response. “We came up with a way to support manufacturers in the vaping industry to retool and get an urgent license to produce it,” he explains. “All the Covid[-19] measures forced us to change focus temporarily, but we never forgot the other challenges our industry was facing.”
Canada’s two major regulatory challenges are nicotine restrictions and flavor bans. David says that those two elements put together—a tobacco-only flavor ban—it would kill the industry. “It’s pretty well over at that point, at least for 90 percent of the industry,” he says. “That’s one of those kinds of things that required us to adjust what we were working on and our priorities as the situation evolved.”

The VITA team believed that fighting the growing number of regulatory challenges was going to require everyone in the industry working together. So, the VITA board and its allies decided to bring together every vape association in Canada in order to share information and coordinate efforts wherever possible. This includes the Canadian Vaping Association (CVA), the only other national vapor trade association in Canada.
“What we’re doing right now is the biggest kind of success story. Even though we might not agree on a lot of things, we still now have a group that meets regularly where we can share intelligence,” says David. “We coordinate some of our efforts so that we’re not working against each other or going in completely opposite directions. Obviously, everybody has their own organization, and they operate independently, but it helps significantly when we’re all talking together, pushing in the same direction on the common ground issues that we face.”
The timing is right. The regulatory challenges won’t be slowing down anytime soon. When the Canadian government released its regulations on limiting nicotine, they stated that [the government] would be looking at banning flavors next, said David. “At a federal level, those two measures, if they were to go through as is, it basically ends the vaping industry in Canada as we know it,” he says. “I think maybe a few shops would survive, but any time you take away 85 [percent to] 90 percent of the products that are the highest margin … and you remove the ability to sell the high-nicotine products, which a lot of people rely on, it makes the whole retail model of a vape shop nonviable.”
It’s a big fight. The VITA response is going to be educational. David said the organization is letting the public know that the government is proposing to make it harder for smokers to switch to a less harmful alternative. David said getting as many people as possible from the industry, including stakeholders and consumers, engaged in this consultation process is vital to the future of vaping.
“The more people that we can get involved and activated by letting the government know that this is not the right approach, the better our chances are,” says David. “That kind of goes with working together with any associations and trade groups. There are a lot of threats coming up. We can’t waste our time fighting each other.”








 Kaival Brands has three new distribution partners for its Bidi Vapor products: Smoker Friendly International, Avail Vapor and Hilmes Distributing. These three additional distributors push the potential U.S. store count for Bidi Vapor products above 46,000, up from 10,000 in 2020.
Kaival Brands has three new distribution partners for its Bidi Vapor products: Smoker Friendly International, Avail Vapor and Hilmes Distributing. These three additional distributors push the potential U.S. store count for Bidi Vapor products above 46,000, up from 10,000 in 2020.








