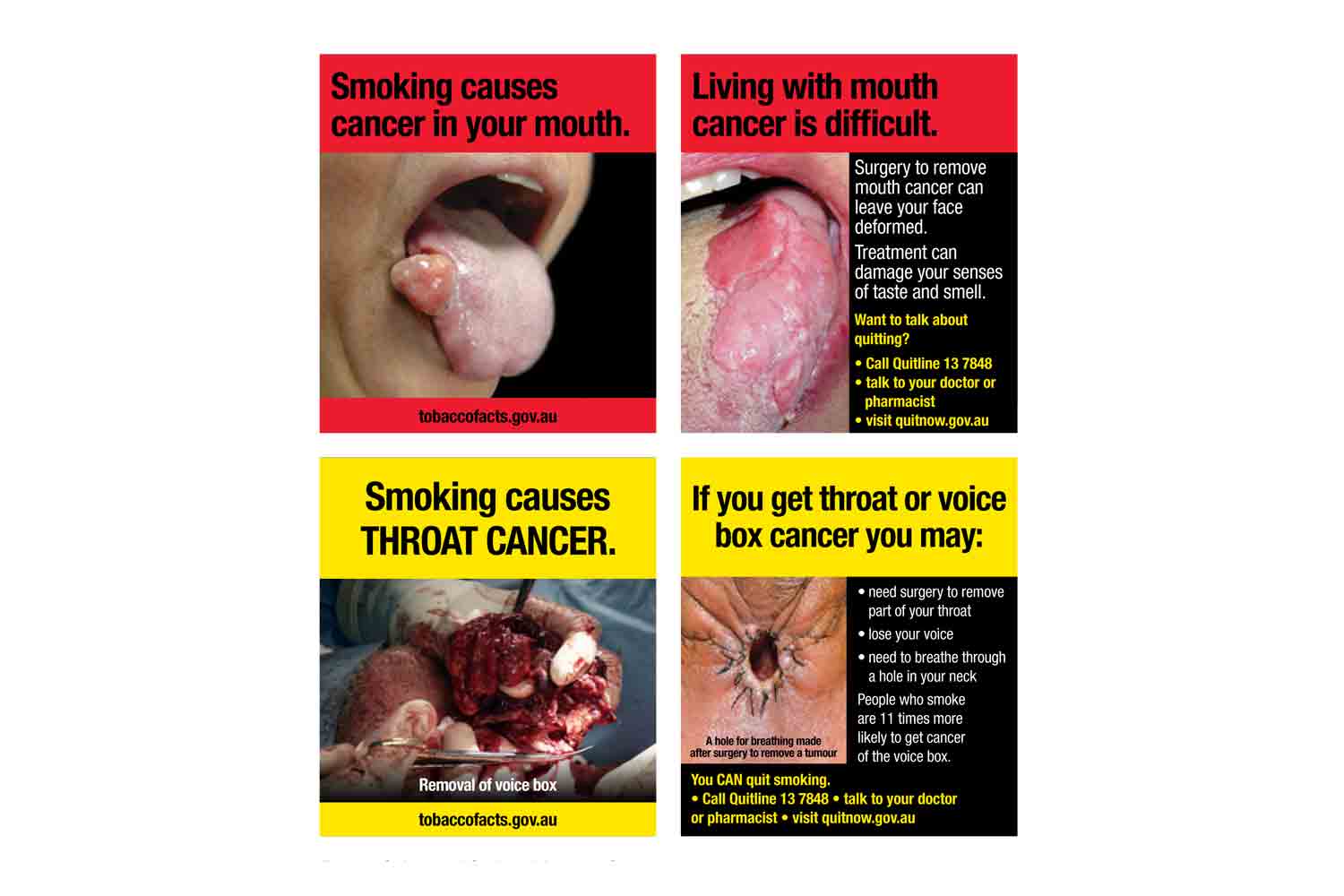
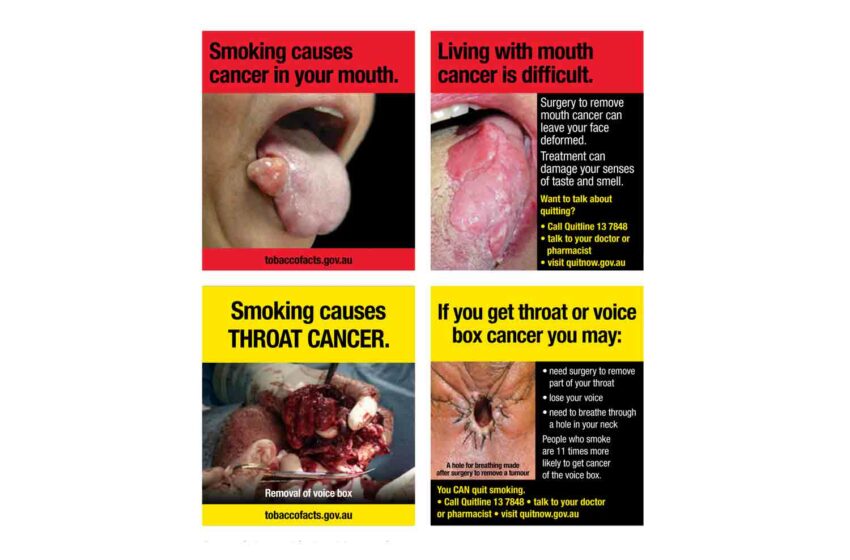
The largest vaping company in the world, Smoore, says that it will no longer partner with brands that use flavor names, packaging or product designs that are attractive to youth.
The announcement comes during the U.K. Government’s consultation on e-cigarettes that has a focus on addressing youth vaping currently underway. The consultation closes on Wednesday.
A press release states that Smoore wants to help end the use of flavor names such as cotton candy, gummy bear, watermelon bubblegum, and blueberry popsicle. Additionally, the company would like to see an end to the manufacturing and sales of “stealth products” which are vaping products designed to mimic school supplies, toys, soft drinks or cartoon characters.
Smoore has created a list of flavors that it considers youth-friendly and is also creating a vapor flavor detection squad to monitor the market for new flavors that could be considered as being appealing to youth.
“The vape industry represents the best chance the world has ever seen to eradicate deadly cigarettes and we cannot allow this opportunity to be squandered,” Rex Zhang, Strategy Smoore’s strategy director, said. “Vaping was invented for this very purpose and we need to ensure that it is focussed on the adult smoking market.
“There is absolutely no place for any vaping product to look like a child’s toy, be shaped like a much-loved cartoon character or iconic children’s game or be filled with liquid called ‘gummy bear, cotton candy, strawberry milkshake or starry violet.”
Every company under Smoore’s umbrella has been ordered to undertake a root and branch review to ensure that none of its products or customers on the OEM and ODM side of its business could be seen as producing youth-appealing products.
The list of flavors so far includes:
- Skittles
- Rainbow
- Cotton Candy
- Donut
- Gummy Bear
- Bubblegum
- Slushy
- Starburst
- Pink Pop
- Ice Cream
- Milkshake
- Popsicle
- Starry Violet
- Reindeer
- Snow
- Christmas
- Fruit Smash
- Dr Reptile
- Sour Patch
- Oreo
- Jolly
If the company finds brand owners with products that Smoore deems to be child-friendly, Smoore will work with the company to take immediate corrective action, however, if no action is taken Smoore could ultimately discontinue all cooperation with the brand.
The Smoore release also suggested a “no-fly list” to be used by retail and distribution companies around the globe that list the manufacturers of child-friendly products to prevent their products from being sold.
“We want other companies to follow our lead on this because we have to ensure that we stop young people vaping and we strongly believe that this must happen regardless of what the government ends up doing,” Zhang said. “We cannot squander this opportunity to help secure a smoke-free generation and, in order to do this, we need both the general public and governments on our side.
“It is only by uniting as an industry from beginning to end and making a clear commitment to doing all in our power to tackle youth vaping that we will be able to achieve this. The UK has always been seen as a world-leading example in fair and proportionate regulation of the vape industry and let’s not give them any reason at all to move from that position.”
Smoore is also calling for more standardization of product sizes and shapes. The company believes standardization will help create faster “disassembly at waste treatment sites, helping to increase recycling rates of vapes.”
The company is calling for every batch of disposable vapes and pre-filled pods to be randomly sampled for product compliance with whole batches being rejected if any number of non-compliant products are identified.
Such measures are necessary to motivate the compliant brands and producers while punishing the offenders,” the release states. “A strict, yet open, marketplace will encourage more innovations in the industry to create products that will serve its job even better with every new generation.”