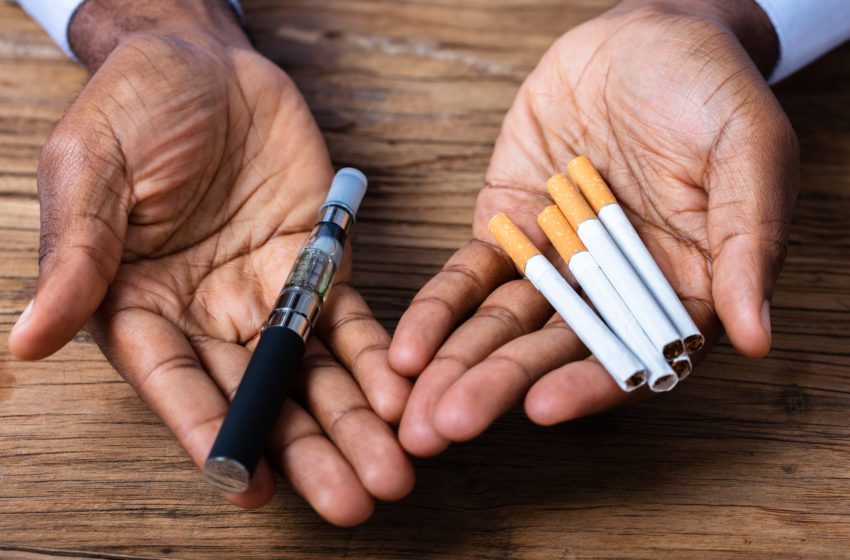
Disposable vapes help smokers to quit combustibles but are deadly for the environment.
By Maria Verven
Cigarettes used to be the most littered things in the world.
Trillions of cigarette butts are thrown onto our streets, parks and beaches every year. The Ocean Conservancy estimates that cigarette butts account for 25 percent of the total number of garbage items collected—over twice as much as any other category. Worldwide, it’s estimated that 1.69 billion pounds of cigarette butts end up as waste each year.
While some smokers may think their butts will eventually decompose, it actually takes decades for them to degrade. Cigarette filters aren’t made of innocuous cotton; they’re made of cellulose acetate and about 12,000 nonbiodegradable plastic-based fibers.
The chemicals in a single cigarette butt can contaminate hundreds of gallons of water. They can also be dangerous, causing fatal fires that burn hundreds of acres every year.
Things have changed dramatically in the last several years as many smokers have switched to vaping, thanks in large part to the convenience of disposable e-cigarettes.
In fact, these handy-dandy devices appear to be taking over the industry since they’re the simplest and most accessible vaping devices on the market.
But in the process, we created a whole new environmental hazard that, as of yet, has no easy solution.
Popular among youth
Among all the vaping devices on the market, none are more popular than disposable electronic nicotine-delivery systems (ENDS), particularly among young people.
According to the 2021 National Youth Tobacco Survey, well over half (54 percent) of youth who reported using e-cigarettes had used disposables. The 2020 Population Assessment of Tobacco and Health Study corroborated this finding. It reported that 38 percent of young adults aged 18–24 versus 17 percent of older adults (over age 25) who had used any ENDS product in the past 30 days had used a disposable.
At the May 2022 Vaper Expo U.K., nearly every vendor offered some variety of disposable device. Many were new to the market that were capitalizing on the trend—as well as renowned companies such as Innokin, which launched its new Aquios Bar disposable device in 10 different flavors.
“Disposable vapes are certainly the hottest-selling item among smoke-free nicotine-delivery devices,” said Dimitris Agrafiotis, owner of Global eVapor Consulting, executive director of the Tennessee Smoke Free Association and brand ambassador and designer at Innokin Technology.
Agrafiotis said disposable vapes attract individuals who make impulse buys at various points of sale as well as new users who enjoy the convenience of a product that doesn’t require any knowledge of coils or ohms. They can purchase disposables nearly anywhere where cigarettes are sold. They can simply tear open the package and start vaping, making disposables the perfect solution for beginners.
“In my experience, vapers who quit smoking use disposable vapes part time as secondary devices when they don’t want to take their usual rig with them, such as at a nice dinner or in situations requiring them to be more discrete,” he said.
The technology behind disposables has only continued to improve over the past several years. Most vape pens can now deliver around 400 puffs before they’re no longer viable—nearly twice as many puffs as a pack of cigarettes can deliver. Some vape pens with larger batteries can even deliver as much as 5,000 puffs.
Another significant advance is the use of auto-draw switches that activate the device and heat the coil when the vaper inhales, delivering a smooth and seamless experience.
And thanks to nicotine salts, disposables offer a smoother vaping experience. While the nicotine level in most disposables is limited to 5 mg, vapers can satisfy their nicotine cravings without a harsh throat hit or any interference in the flavor experience.
Speaking of flavor, that’s another advantage disposables have over refillable vape devices. Manufacturers often add sweeteners to disposables to make the flavors pop without having to worry that the sweeteners will gunk up and ruin the device. The disposable will be tossed long before that happens.
The range of flavors available from disposables is mind-blowing. As more and more manufacturers take advantage of the growth in this market, they entice vapers with interesting and often exotic flavor profiles, such as bergamot and carambola.
While battery technology hasn’t necessarily improved dramatically, some brands have created larger internal or rechargeable batteries in their efforts to increase puff count. This is a step in the right direction to reduce battery waste.
The environmental impact
Even refillable and replaceable vape pens typically contain several metal, plastic and cotton elements, making them difficult to separate and recycle. Thus, they tend to end up as general household waste. Even the smaller replaceable coils and pods don’t often get recycled.
But disposable e-cigarettes are way worse because the vaper disposes the entire device, which is composed of plastic and metal coils as well as a battery cell. While some brands and vape stores offer recycling programs for disposables, most vapers simply toss them into the trash.
Millions of lithium-ion batteries, hard plastic and nicotine-contaminated pods are being disposed of in our landfills, creating a significant waste problem. Nicotine, including nicotine salt, is listed by the Environmental Protection Agency as an acute hazardous waste. When disposables leak battery acid and/or nicotine into the environment, they harm fish and wildlife in the process.
The Food and Drug Administration is required under the National Environmental Policy Act to evaluate all major agency actions to determine if they will have a significant impact on the human environment. If the environmental assessment identifies significant environmental effects, the FDA will prepare an environmental impact statement to help make informed decisions on the relevant environmental consequences and alternatives available.
In addition to assessing potential environmental impacts of new tobacco products during premarket review, the FDA has also posted information for consumers on proper disposal of e-cigarettes and e-liquid waste.
“While we are excited that lots of people are not inhaling combustible tobacco, we should be concerned over the environmental sustainability and proper ethics in the sale of these products,” Agrafiotis said. “In its quest to market and sell millions of these products, the industry has failed to implement any type of consumer education or recycling initiative that would help alleviate the disaster,” he said.
“The irony is that in most countries in Europe, plastic straws are banned—and yet these products continue to be dumped by the boatloads. I simply cannot see how governments will allow this to continue, especially in Europe, where environmental waste is such a huge issue,” Agrafiotis said.
“With TPD 3 approaching and countries already discussing legislative measures, I believe the days are numbered for disposables—at least as we know them right now.”
What’s the solution?
The first and most obvious answer is to encourage consumers to use rechargeable devices.
Consumers could also be encouraged to purchase refillable pod devices, vape pens with replaceable coils or even rebuildable tank atomizers, all of which are far more cost effective in the long run, not to mention more eco-friendly.
The industry has yet to find ways to encourage and/or incentivize consumers to dispose of these devices in the right manner. When Agrafiotis tried offering a financial incentive for every disposable brought back to his store, there were very few takers.
“The younger demographic that predominantly uses these products simply doesn’t seem to care,” he said. “At least the older demographic tends to quickly transition from disposables to open systems when they realize the daily costs and environmental impact.”
Agrafiotis said he’s unaware of any other outlets for collecting and recycling disposable vapes. “At this point, there’s no budget or avenue for us to try and change the existing system. Incentives and/or drop-off points for hazardous waste should have started with the construction and sale of the first disposable vaping device ever made.”
“The only thing I could do is break the plastic and remove the battery and bring it to a battery recycler, but I would still have to dispose the plastic and nicotine pod in the trash,” he said. “All brands would have to work together to start a viable recycling program, but unfortunately, I simply do not see this is possible.”
Nevertheless, Agrafiotis said Innokin is striving to reduce environmental waste in its products. Innokin was the first company to start using fully recyclable packaging for its open vapor systems, made entirely of paper with absolutely no plastic, he said.
The first disposable vaping device that can be disassembled and recycled, the Innokin Enviro uses materials with a lower carbon footprint—a reinforced paper shell—to replace the plastic shell found in most disposable vaping devices.
“We believe disposable vapes should have less impact on the environment,” Agrafiotis said. “With more efficient manufacturing processes and recyclable designs, our goal is to continually optimize Enviro and make disposable vaping greener. We can only hope demand grows for this approach and more companies follow in the same green footsteps.”
Clearly, the industry must act quickly to devise solutions before the products that help millions of smokers are carbon taxed or—even worse—removed completely from the market.
“Most of all, I hope we see more people quit smoking and transition to vaping, regardless of the device they choose to help them. Any vaping devices that can help smokers around the world make the switch is worth pursuing,” Agrafiotis said.
“Plastic casings and batteries simply should not go into our landfills after just one use,” he said. “More companies should be actively looking at sustainable solutions and proactively working with existing recycling companies to implement programs to keep these products out of our already overflowing landfills.”
The original “Vaping Vamp,” Maria Verven owns Verve Communications, a PR and marketing firm specializing in the vapor industry.
MORE ON VAPING WASTE
Garbage facts
There is an estimated 44.7 million tons of e-waste generated around the world every year. That waste contains up to $65 billion worth of raw materials like gold, silver and platinum sent to a landfill. The amount of global e-waste is expected to increase by almost 17 percent to 52.2 million tons in 2021, or about 8 percent every year, according to Cleanaway Waste Management, an Australian waste management, industrial and environmental services company.
Vaping products contain lithium-ion batteries, a heating element and a circuit board. These components—which may include plastic and heavy metals—make disposing of e-cigarettes a considerable challenge because of the various types of chemicals and materials involved in their manufacturing.
The global disposable e-cigarettes market size is expected to be valued at $6.34 billion in 2022, according to Future Market Insights (FMI). The overall demand for disposable e-cigarettes is projected to grow at a CAGR of 11.2 percent between 2022 and 2032, totaling around $18.32 billion by 2032.
“Demand for non-tobacco products is expected to augment the growth of the disposable e-cigarettes market in the near future. It has been observed that older people prefer this product as it does not have any negative effect on health,” stated an FMI analyst.
There are no direct regulations for recycling or use of e-cigarettes, heated-tobacco products (HTPs) or the cellulose acetate filters in combustible cigarettes in the EU, U.S., China and Japan. There is some legislation that regulates the management of e-waste; however, these guidelines typically apply only to cell phones, computers and other large electronic products.

According to the Global Overview of Recycling Programs for E-Cigarettes, Heated-Tobacco Products and Vaporizers Business for 2022 and Future Prospects of Electronic Devices and Consumables Development report by Research and Markets, large vaping industry players have several recycling programs and recycling targets for the near future:
- Philip Morris International established two hubs in Europe and Asia that inspect, process and separate materials from electronic devices for recycling. The effective recycling rate of IQOS devices increased from 30 percent in 2018 to 40 percent in 2020. The target recycling rate is 80 percent by 2025.
- BAT replaces plastic elements of vapor products with pulp-based alternatives. The share of recycled waste was 79–80 percent in 2019–2021. The target recycling rate is 95 percent by 2025.
- Japan Tobacco International launched a return scheme of used devices through the recycling boxes at shops. In 2020, 67 percent of produced waste was recycled. The target for waste reduction is 20 percent by 2030.
- Imperial Brands launched takeback recycling schemes for used vaping devices and pods. The recycling rate decreased from 69 percent in 2017 to 61 percent in 2021. The target recycling rate is 75 percent by 2030.
- Other vape companies (Dotmod, Shanlaan, Dovpo and Vinn) launch their own recycling programs by return schemes. Innokin works on battery utilization programs.
- FEELM, an atomization brand and an independent business unit of Smoore Technology Ltd., won the IF Design Award 2020 for its eco-friendly Disposable Paper E-cigarette. CCELL launched a new line of disposable vaporizers in 2021.
- Recycling companies Gaiaca and TerraCycle cooperate with vape manufacturers to provide services for collecting and recycling e-waste. Some vape producers cooperate directly with recycling companies; for example, RELX cooperates with China Siyan Foundation for Poverty Alleviation.
- The Bowman Company offers refill stations to fill empty vapor bottles/pods. It will help to reduce plastic usage for vapor bottle production in the future.
It is expected that the future of e-cigarette, HTP and vaporizer recycling will depend on producers’ product life cycle programs. Recycling decisions from large vaping companies to combat waste include using a combination of polylactic acid (PLA) and plastic or starch blend and plastic for the device body; using paper packaging; and making inner packaging consist of paper or paper and PLA.
A survey by Opinium on behalf of Material Focus, a not-for-profit established to help the U.K. meet its electrical reuse and recycling targets, found that 18 percent of 4,000 people surveyed in the U.K. had bought a vape device in the previous year, with 7 percent buying a single-use device.
The Opinium figures would suggest that about 168 million disposable vapes are being bought every year in the U.K. Two of the biggest brands in the country are Elf Bar and Geek Bar, which between them make up about 60 percent of the market.
More than half of people that buy single-use e-cigarettes dispose of them in a general trash bin compared to 33 percent on average for all types of vape, according to the research. While each vape contains just 0.15 g of lithium, the scale of the waste means that about 10 tons of metal is ending up in landfills. – VV staff