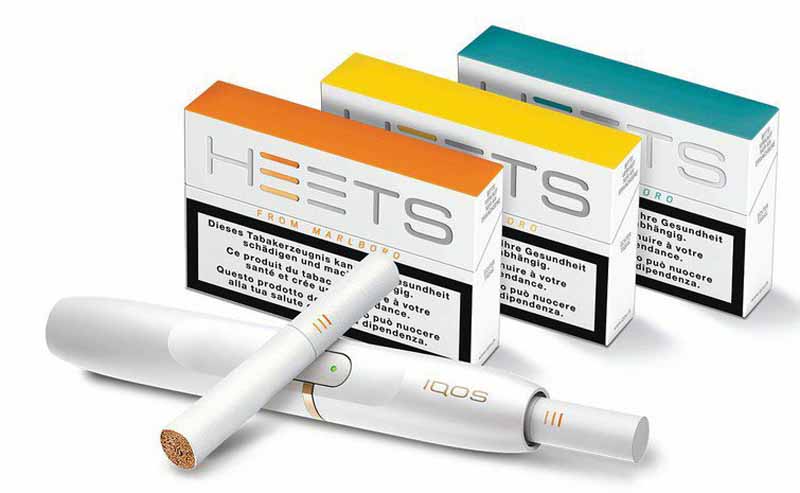
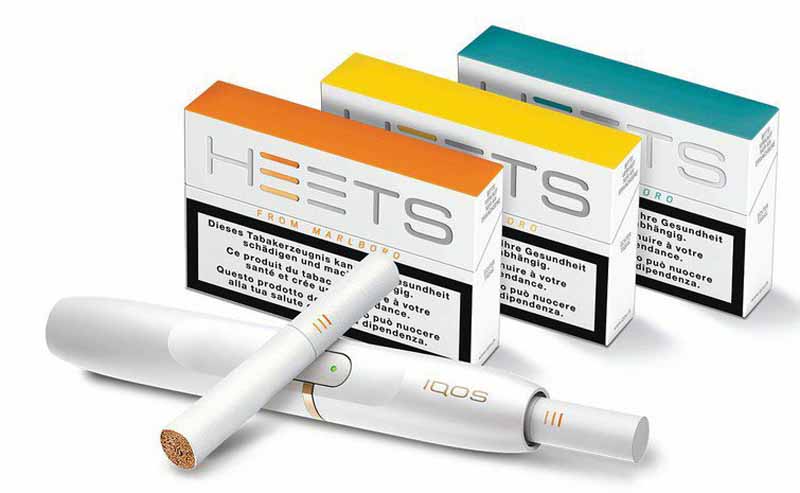
Philip Morris International has launched IQOS Iluma, the brand’s first tobacco-heating system based on induction-heating technology, in Japan.
The device’s Smartcore induction system heats the tobacco from within the new Terea Smartcore Stick. These newly designed sticks are to be used only with IQOS Iluma, which features an auto-start function that detects when the Terea stick is inserted and automatically turns on the device.
According to PMI, the bladeless IQOS devices offer a cleaner way to heat tobacco from the core without burning it. They also provide a more consistent experience and leave no tobacco residue, eliminating the need to clean the device. Additionally, the devices generate no combustion and no smoke. PMI says its market research indicates that IQOS Iluma provides a more pleasurable experience compared to previous IQOS generations.
“IQOS Iluma is our most innovative offering to date and the new flagship in our portfolio of science-backed, smoke-free products. Its breakthrough induction-heating technology heats tobacco from within, without burning, so there’s no smoke, no ash and, like previous IQOS devices, it emits, on average, 95 percent lower levels of harmful chemicals compared with cigarettes,” said Michele Cattoni, vice president of heated-tobacco platforms at PMI, in a statement.
“However, unlike our previous tobacco-heating systems, IQOS Iluma has no blade. That means no tobacco residue or cleaning—ever. With this, and other product features, we aim to address consumer pain points that may have hindered some adult smokers from beginning or maintaining their journey away from cigarettes in the past.”
IQOS Iluma is available in two versions—IQOS Iluma Prime and IQOS Iluma. Both devices use new induction-heating technology but offer different designs. IQOS Iluma Prime and IQOS Iluma are available in Japan for pre-order on IQOS.com beginning Aug. 17, 2021, and for purchase at IQOS stores on Aug. 18, 2021.
As of June 30, 2021, PMI’s smoke-free products are available in 67 markets. The company has stated its ambition to be present in 100 markets with its smoke-free products by 2025. There are more than 20 million users of the IQOS tobacco-heating system globally, and PMI estimates that more than 73 percent (approximately 14.7 million) of these men and women have switched completely to IQOS and stopped smoking with the balance in various stages of switching. PMI’s ambition is that by 2025, at least 40 million PMI cigarette smokers who would otherwise continue to smoke will have switched to smoke-free products. Furthermore, the company’s ambition is that more than half of its net revenues will come from smoke-free products by 2025.