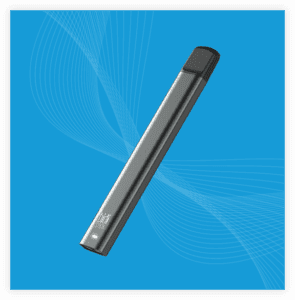
Kaival Brands Innovations Group, the U.S. distributor of all Bidi Vapor products, has entered into a sales broker agreement with a prominent U.S. broker to expand access to Bidi Vapor products from its current foundation of convenience store distribution into new retail channels, including discount, grocery and mass merchandisers.

“As we look to push distribution into more channels beyond the convenience stores, we are excited to announce a new agreement that gives us potential access to over 40,000 new locations,” said Eric Mosser, president and chief operating officer of Kaival Brands, in a statement. “We believe this agreement, along with our recent announcement of other new distribution agreements, further validates our reputation as a good actor providing adult consumers with the highest quality vape experience possible, and we look forward to working with all of our commercial channel partners to expand our revenue opportunities.”
“We are excited to further increase the reach of Bidi Vapor and its premium vaping device, the Bidi Stick, into potentially more distribution opportunities throughout multiple retail channels,” stated Russell Quick, president of QuikfillRx, the company’s third-party sales and marketing vendor. “With our feet firmly in the convenience store space, it is time not only to grow our existing footprint but to extend into more channels, like dollar and grocery stores, that meet our robust identification verification and youth access prevention requirements.”