Health groups are urging the U.S Food and Drug Administration to make haste in addressing the remaining premarket tobacco applications (PMTA) for leading e-cigarette brands such as Juul, Blu, Vuse (Alto) and NJOY, which make up 75 percent of the U.S. market and are among the most popular with youth.
“We are grateful to see movement again by the FDA on the e-cigarette pre-market approval process under Dr. Califf’s leadership and the recognition of the risks these products pose to America’s youth while assessing the public health benefit for adult smokers,” wrote Robin Koval, president and CEO of the Truth Initiative, in a statement following the FDA’s authorization of Logic Technology Development’s tobacco-flavored e-cigarettes. Logic, however, makes up a small percentage of the U.S. e-cigarette market, with just over 1 percent according to retailer scanner data.
The Truth Initiative also expressed concern about the FDA’s failure to deny marketing applications for Logic’s menthol e-cigarettes, which remain under review. “According to the latest NYTS data, nearly 30 percent of young people who use e-cigarettes reported using a menthol flavor,” wrote Koval. “As the FDA prepares to issue a proposed rule removing menthol cigarettes from the market, we continue to urge the FDA to remove all flavored tobacco products, including menthol to protect our nation’s youth.”
Meanwhile, tobacco harm reduction advocates, took the approval of Logic, which is ultimately owned by Japan Tobacco International, as further evidence that the PMTA process favors deep-pocketed tobacco multinationals.
“It’s mildly gratifying to hear FDA say out loud the obvious and simple truth that vaping is helping Americans quit smoking,” Amanda Wheeler, president of the American Vapor Manufacturers Association told Filter. “But meanwhile in the bureaucratic shadows, they are strangling the life out of our entire innovative, entrepreneurial industry.”
“The FDA should be thoroughly embarrassed that the only vaping products with PMTAs are ones that have been rejected by adult consumers,” Greg Conley, the president of the American Vaping Association, told Filter. “If JTI did not have cigarette sales to subsidize their minimal effort offerings in next-gen products, market forces would have caused them to stop selling these products years ago.”









 “Although we are not surprised to learn that Japan Tobacco Inc., brand owner of Logic, is now among the Big Tobacco companies with FDA market authorization, we certainly aren’t pleased with FDA’s consistent rejection of flavored products and will continue to apply pressure in that regard, as well as in the enforcement discretion arena – particularly for the manufacturers with products still in review that participate in our Responsible Industry Network program,” said April Meyers, CEO of the Smoke-Free Alternatives Trade Association (SFATA). “As the nation’s leading regulatory body, the agency appears to be cherry-picking what science it utilizes for decision making. That FDA cited the recent NYTS data but failed to acknowledge the steep decline in youth use while coining the low rates an “epidemic”, makes its rejection of flavored products today seem more an act of fear over what might happen than a decision based on scientific evidence. This is disappointing, at best, but again, not surprising.”
“Although we are not surprised to learn that Japan Tobacco Inc., brand owner of Logic, is now among the Big Tobacco companies with FDA market authorization, we certainly aren’t pleased with FDA’s consistent rejection of flavored products and will continue to apply pressure in that regard, as well as in the enforcement discretion arena – particularly for the manufacturers with products still in review that participate in our Responsible Industry Network program,” said April Meyers, CEO of the Smoke-Free Alternatives Trade Association (SFATA). “As the nation’s leading regulatory body, the agency appears to be cherry-picking what science it utilizes for decision making. That FDA cited the recent NYTS data but failed to acknowledge the steep decline in youth use while coining the low rates an “epidemic”, makes its rejection of flavored products today seem more an act of fear over what might happen than a decision based on scientific evidence. This is disappointing, at best, but again, not surprising.” “People forget that in the story, Dr. Jekyll was a benevolent physician in a lab coat who only wanted to help people. But tomorrow morning, (FDA Commissioner) Robert Califf and (director of the FDA’s Center of Tobacco Products) Mitch Zeller will transform back into their Mr. Hyde alter-egos and resume their hellbent mission to sabotage the single-most effective smoking cessation device ever devised,” said Wheeler. “Well, the American people are watching and I for one am not going to stand by and let them get away with it. So, here’s my own announcement for today: FDA and CDC have my approval to stop deceiving the American public about the safety and efficacy of nicotine vaping.”
“People forget that in the story, Dr. Jekyll was a benevolent physician in a lab coat who only wanted to help people. But tomorrow morning, (FDA Commissioner) Robert Califf and (director of the FDA’s Center of Tobacco Products) Mitch Zeller will transform back into their Mr. Hyde alter-egos and resume their hellbent mission to sabotage the single-most effective smoking cessation device ever devised,” said Wheeler. “Well, the American people are watching and I for one am not going to stand by and let them get away with it. So, here’s my own announcement for today: FDA and CDC have my approval to stop deceiving the American public about the safety and efficacy of nicotine vaping.”
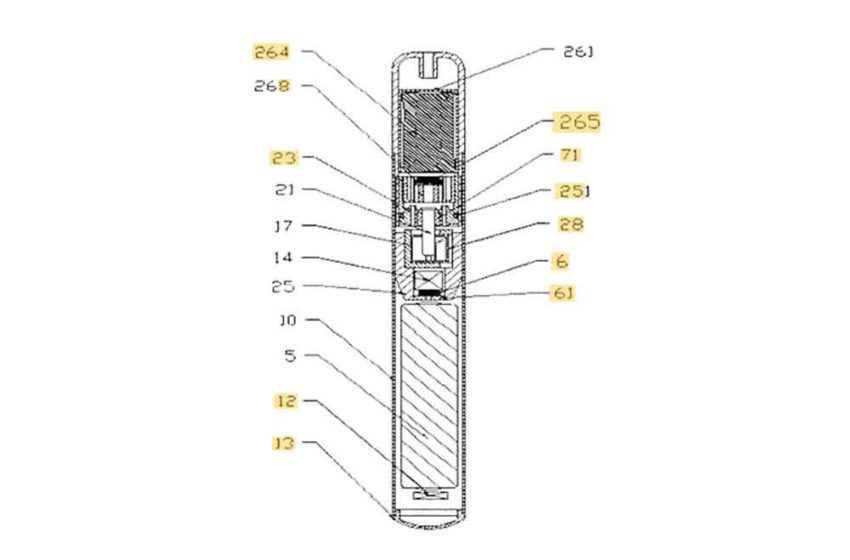
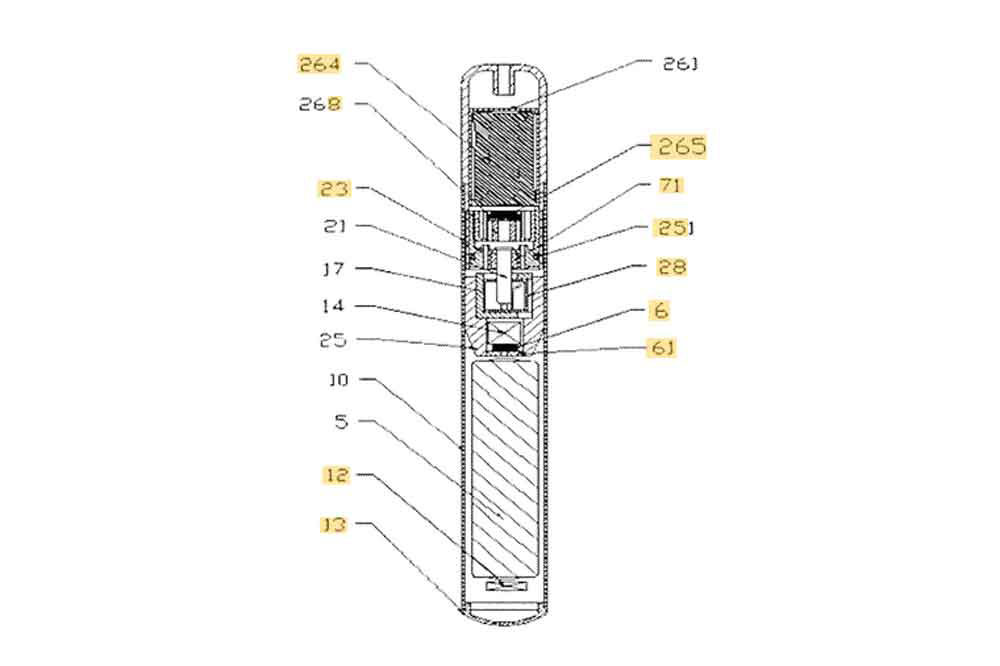
 Vape manufacturer GeekVape, the parent of Geek Bar, has written to trading standards departments in major UK cities asking them to take more action to combat the growing market for illicit vaping products.
Vape manufacturer GeekVape, the parent of Geek Bar, has written to trading standards departments in major UK cities asking them to take more action to combat the growing market for illicit vaping products.