The arrival of IQOS in the U.S. market raises some questions and eyebrows.
By Maria Verven
After a two-year wait, Philip Morris International (PMI) has received the go-ahead from the U.S. Food and Drug Administration (FDA) to sell its IQOS heat-not-burn (HnB) tobacco product in the U.S. Reportedly, an estimated 7.3 million consumers worldwide have already switched from combustible cigarettes to the IQOS.
The whole idea is that when tobacco is heated and not burned as in traditional cigarettes, the level of harmful chemicals is much lower. Because the IQOS uses tobacco, PMI claims it’s “a closer experience to smoking cigarettes than other alternatives such as e-cigarettes.”
What exactly is this new product? How does it work? And what possible effect will it have on the U.S. vapor market?
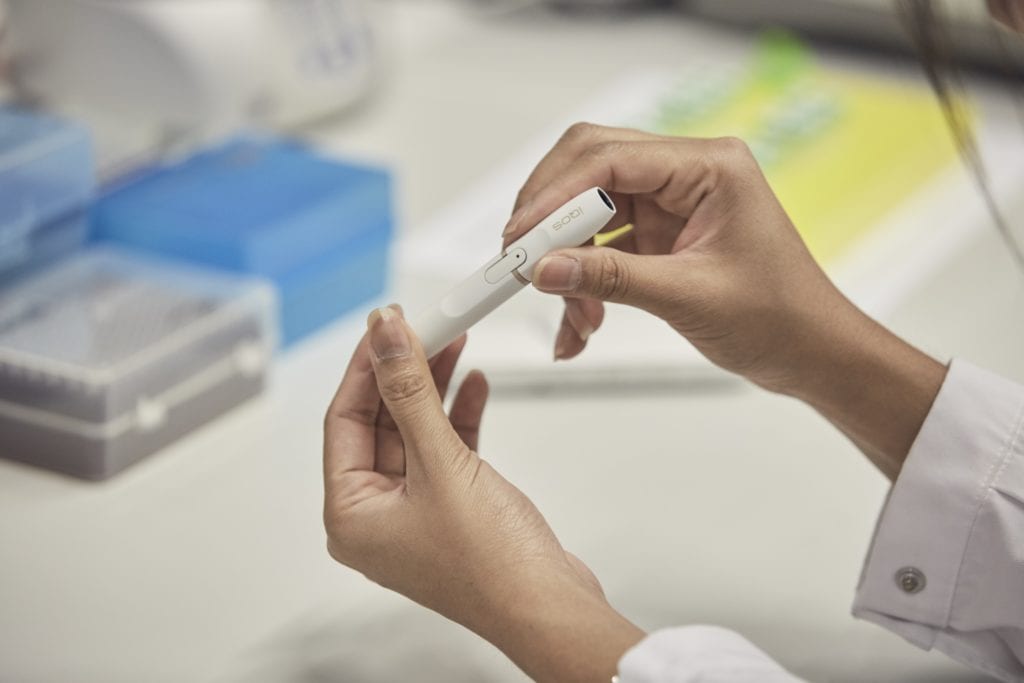
What’s an IQOS and how does it work?
The IQOS (pronounced EYE-kose) works by heating tobacco-filled sticks called HeatSticks just enough to release nicotine-containing vapor (up to 350 degrees C or 662 degrees F) but without creating combustion, fire, ash or smoke.
The IQOS comes in two different versions: One contains three parts—HeatSticks, the IQOS holder and a charger, and the other combines the holder and charger, enabling the user to use it multiple times before having to recharge the battery. Both work the same way: You insert a HeatStick into the IQOS, push a button to turn on the electronically controlled heater and then draw on the end of the HeatStick, which releases an aerosol and the taste of tobacco.
When you’re done, the HeatStick goes in the trash while the IQOS goes back into the charger. The integrated version lasts for 10 consecutive uses before it needs to be recharged.
What’s a HeatStick?
HeatSticks, or HEETS, contain tobacco “plugs” made from tobacco leaves that have been ground and reconstituted into tobacco sheets. Called “cast-leaf,” these sheets are crimped and made into tobacco plugs. They’re specifically designed to be heated, not burned as in traditional cigarettes. Regular cigarettes don’t work in the IQOS.
How much does the IQOS cost?
IQOS kits retail starting at $116 (IQOS 2.4 kits are available on Amazon) and up to $300 for the IQOS 3. One carton (10 individual packs of 20 HeatSticks), available in Marlboro, Marlboro Smooth Menthol and Marlboro Fresh Menthol, costs around $90.
Where will it be marketed?
IQOS has been sold in cities all over the world, including in Canada, Europe, Japan and Russia. Now that PMI has secured FDA approval, PMI’s parent company, Altria, will open a brick-and-mortar store as well as mobile stores in Atlanta, Georgia, USA. Shortly thereafter, they will begin selling IQOS all across the U.S., starting in about 500 retail stores, including Circle K, Murphy USA, QuikTrip, RaceTrac and Speedway.
“Japan has had IQOS and similar products longer than any other major market, and in a mere three years, Japan’s cigarette sales fell by a third,” says David Sweanor, chair of the advisory board for the Centre for Health Law, Policy and Ethics at the University of Ottawa. Sweanor has worked on public health policy for tobacco since 1983.
“Scandinavian countries, the U.K. and other places are all clearly showing the ability to replace combustibles. We have never seen such a dramatic decline in smoking,” Sweanor said, predicting that the market will respond with an increasing array of ever-better alternatives to cigarettes, just as risk-reduction innovations have transformed a vast range of other goods and services.
Are there other heat-not-burn products on the market?
Big tobacco companies—which realized as early as the 1950s that cigarettes were potentially lethal—have been trying for decades to develop technology that eliminates the combustion of tobacco since that’s when carcinogens are created.
The idea to create an HnB tobacco product has been unsuccessfully tested in the market at least since 1988 when R.J. Reynolds Tobacco Co. (RJR) came out with Premier. Thus far, these products have not been able to gain market traction, even after constant redesigning and rebranding.
For example, Premier was later redesigned and rebranded as Eclipse, which was sold for only four years, from 2003 to 2007. After that product failed to catch on, RJR came out with Revo, which was just a redesigned version of Eclipse. After that product flopped, it was pulled off the shelves in 2015.
PMI came out with its first HnB product called Accord in 1998, but it too failed to gain traction and was pulled from shelves around 2006. Marketed as a “cleaner” tobacco product, PMI claimed that Accord reduced exposure to the harmful compounds normally released by burning tobacco.
Critics contend that the IQOS is nothing new. The IQOS is a variation on the Accord “without consistent improvements in exposure to aerosol toxic compounds,” says Stanton A. Glantz, director for the Center for Tobacco Research Control and Education at the University of California in San Francisco, California, USA.
“In contrast to PMI’s past claims for Accord, PMI now claims in its MRTP [modified-risk tobacco product] application that IQOS reduces health risk,” Glantz wrote. “This shift in stance is likely not the result of any toxicological difference between Accord and IQOS but rather a change in the social and regulatory landscape permitting these claims.”
Are youth using the IQOS?
In response to a much discussed “epidemic” of teen vaping (see “FDA in Transition,” Vapor Voice, Issue 2, 2019), the FDA has cracked down on e-cigarette sales and marketing. In response to a lawsuit by the American Academy of Pediatrics and other health groups, a federal district judge recently ordered the FDA to speed up the timeline for reviewing the thousands of vapor products now on the market.
Nevertheless, the FDA and PMI both claim that the IQOS won’t be as popular among teenagers as e-cigarettes since it doesn’t come in sweet or fruity flavors. Plus, the price is much higher, especially compared to Juul pods, which have become the most popular vapor product on the market. However, the FDA will still require IQOS advertisements be targeted at adults and will prohibit television and radio advertising.
“Given the rapid pace of innovation in the tobacco products space, we are emphatic that youth should not use any tobacco- or nicotine-containing product,” said PMI’s CEO, Andre Calantzopoulos. “Youth should not become nicotine users. PMI takes that responsibility seriously. In our IQOS stores, we refuse to offer these products to people who have never smoked or those who have quit smoking. We are also clear that these products are not risk-free or a safe alternative to cigarettes.”
“There is a balance that must be struck,” he continued. “Public policy needs to recognize the role that new smoke-free tobacco and other nicotine-containing products can play in helping move adult smokers away from cigarettes. Achieving this balance is absolutely necessary to realizing a true public health breakthrough and requires close coordination with the regulatory agencies.”
Calantzopoulos said that real-world data from countries where IQOS is currently being sold shows that it is reaching the correct audience—current adult smokers. For example, in Japan, the largest IQOS market thus far, 98 percent of IQOS users were tobacco users before switching. Globally, the average IQOS user is between the ages of 30 and 49.
Is the IQOS safer than combustible cigarettes? Where does it fit on the risk continuum?
Although PMI claims that using IQOS exclusively will significantly reduce harm from smoking traditional cigarettes, the FDA is still reviewing PMI’s modified-risk tobacco product application that would allow it to market IQOS as being safer than cigarettes.
PMI’s website touts the following clinical finding: “Smokers switching to Platform 1 (i.e., IQOS) were exposed to lower levels of harmful chemicals compared to those who continued smoking. We measured biological markers in the blood and urine and found that levels of exposure to harmful and potentially harmful constituents (HPHC) were comparable to the levels of those who quit smoking for the duration of the study.”
The FDA corroborates PMI’s claim that levels of cancer-causing chemicals are lower in IQOS’ aerosol than in cigarette smoke. In the news release announcing its approval of the premarket tobacco product application for IQOS, the FDA states, “Through the FDA’s scientific evaluation of the company’s applications, peer reviewed published literature and other sources, the agency found that the aerosol produced by the IQOS tobacco-heating system contains fewer toxic chemicals than cigarette smoke, and many of the toxins identified are present at lower levels than in cigarette smoke. For example, the carbon monoxide exposure from IQOS aerosol is comparable to environmental exposure, and levels of acrolein and formaldehyde are 89 percent to 95 percent and 66 percent to 91 percent lower than from combustible cigarettes, respectively.”
Overall, HnB products have been the source of much study. A systematic literature review of 31 publications on HnB products, secondhand emissions and human exposure published in September 2018 in Tobacco Control found that compared with cigarettes, HnB products reduced levels of HPHC by at least 62 percent and particulate matter by at least 75 percent.
However, it also found that while “HnB use suppressed urges to smoke, participants rated HnB [products] less satisfying than cigarettes. In addition (and in comparison to e-cigarettes), HnB products exposed users and bystanders to toxicants, although at substantially lower levels than cigarettes.”
Not surprisingly, public health groups aren’t any more enthusiastic about HnB products than they are about vapor products, as they remain entrenched in an abstinence-only stance. American Lung Association CEO Harold Wimmer in a statement said that his organization is “deeply concerned about the health impacts of this new product.”
Advocates of the vapor market are also unimpressed.
“If e-cigarettes and vaping products did not exist, then IQOS would be a valuable addition to the market,” said Michael Siegel, professor at the Department of Community Health Sciences at the Boston University School of Public Health. “However, since we already have a thriving market of vaping products—which do not contain any tobacco and are orders of magnitude safer than cigarettes—I don’t see how a heat-not-burn tobacco product really advances the smoking cessation product market.
“There are much safer alternative nicotine-delivering products. While IQOS is somewhat safer than smoking, it is still far more hazardous than vaping products. On the risk continuum, with e-cigarettes at the low end and cigarettes at the high end, IQOS would probably be up towards cigarettes, though not as high but still way above the risk of e-cigarettes.
“To the extent that IQOS diverts attention away from e-cigarettes, it could have negative public health effects. Our focus should be on those vaping products,” Siegel said. “The best scenario would be if IQOS attracts smokers who want to quit but were not able to quit using e-cigarettes or any other strategies.”