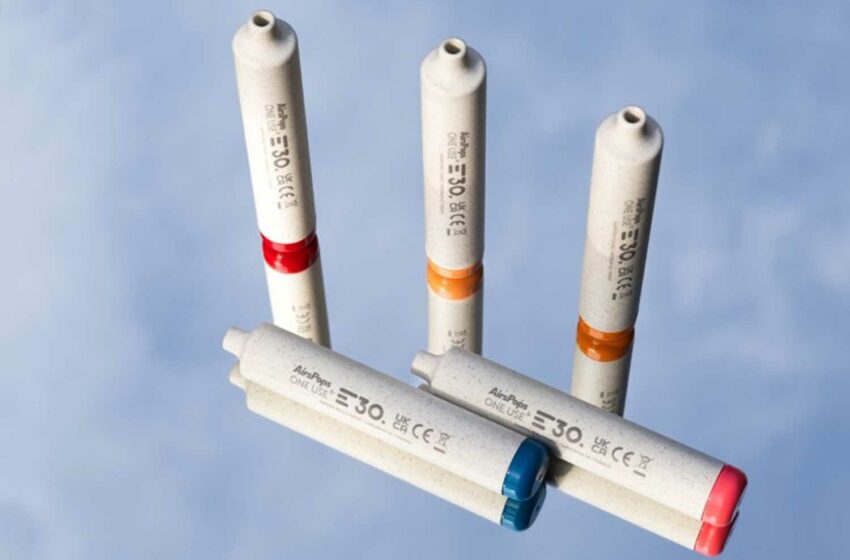
The resolution of an IP dispute with BAT has removed a major hurdle to selling the product in the U.S.
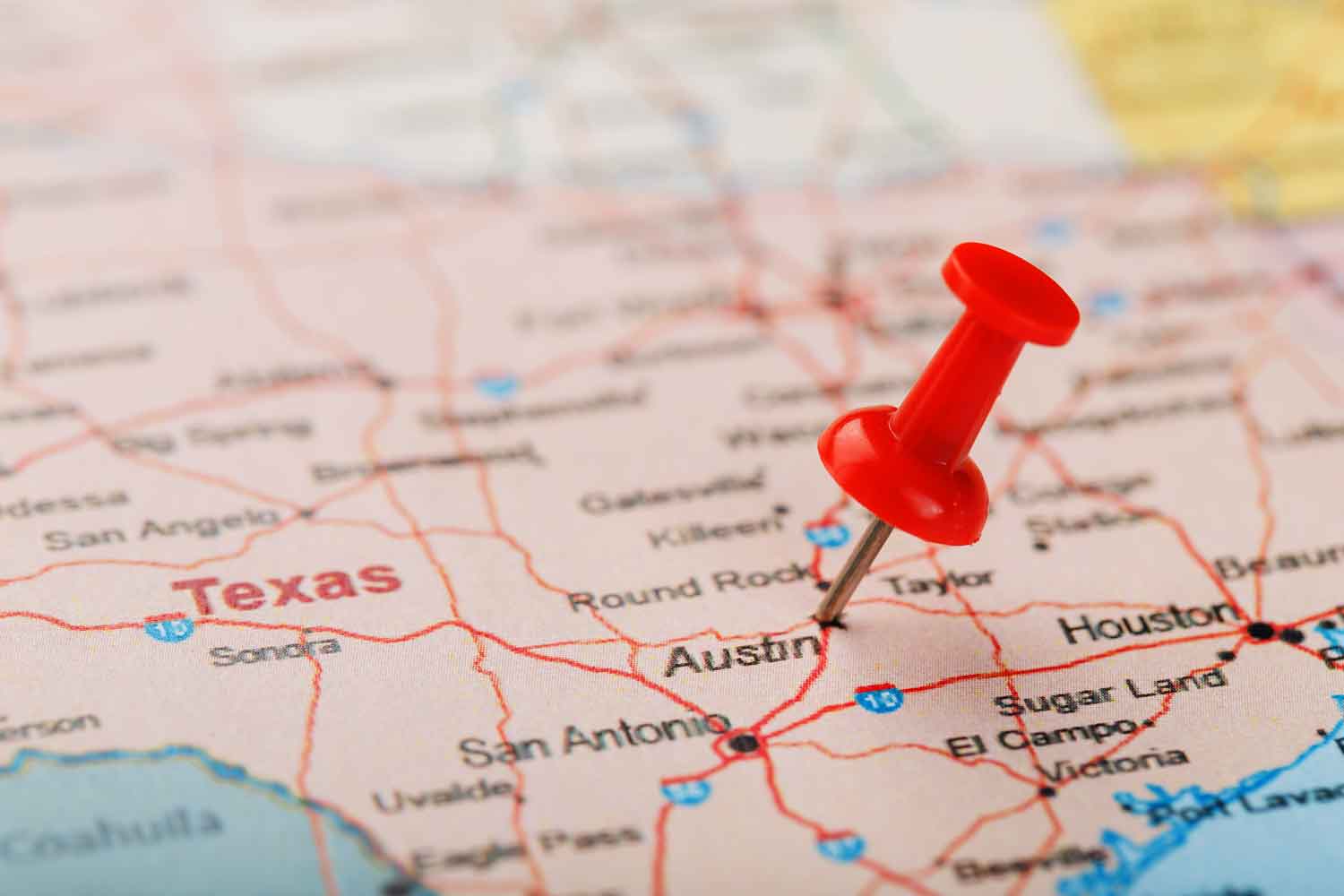
Philip Morris International is preparing to launch its IQOS heated-tobacco device in Austin, Texas, USA, reports U.S. News. The city will be a testing ground for PMI’s re-entry into the United States after the company resolved an intellectual property dispute with British American Tobacco that had prompted the International Trade Commission to ban imports of IQOS in the United States.
PMI previously announced that it planned to launch IQOS in four cities in two U.S. states beginning with one city in the second quarter before a larger rollout in 2025. The company did not, however, release details.
According to U.S. News, LinkedIn job advertisements suggest that PMI is planning to launch the product in Austin. The advertisements were posted this month and include positions such as field sales representatives, territory managers and retail sales advisors.
The U.S. would be a significant market for IQOS. Euromonitor estimates that total U.S. nicotine sales excluding nicotine-replacement therapies were $143.6 billion in 2022. Cigarettes accounted for the majority of sales, but Euromonitor predicts that their value will drop by 30 percent by 2027 and the value of smoking alternatives such as e-cigarettes and nicotine pouches, will increase by 36 percent in the same period.
Investors are waiting to see if PMI can create a heated-tobacco market in the U.S., where vaping is dominant.
According to Brett Cooper, managing partner and analyst at equity research firm Consumer Edge, Texas offers an interesting trial market due to broad demographics. He noted that diverse cities like Austin, Houston and Dallas provide access to a wide range of consumer groups.
U.S. Centers for Disease Control and Prevention data shows that tobacco taxes in Texas are relatively low, with the excise tax rate on a pack of cigarettes standing at $1.41 in September 2023.
In January, Texas introduced new e-cigarette laws, banning products that resemble food or that include symbols or celebrities targeted at minors or that depict cartoon-like fictional characters.
PMI believes IQOS can capture a 10 percent share of the U.S. tobacco and heated-tobacco unit volume by 2030.