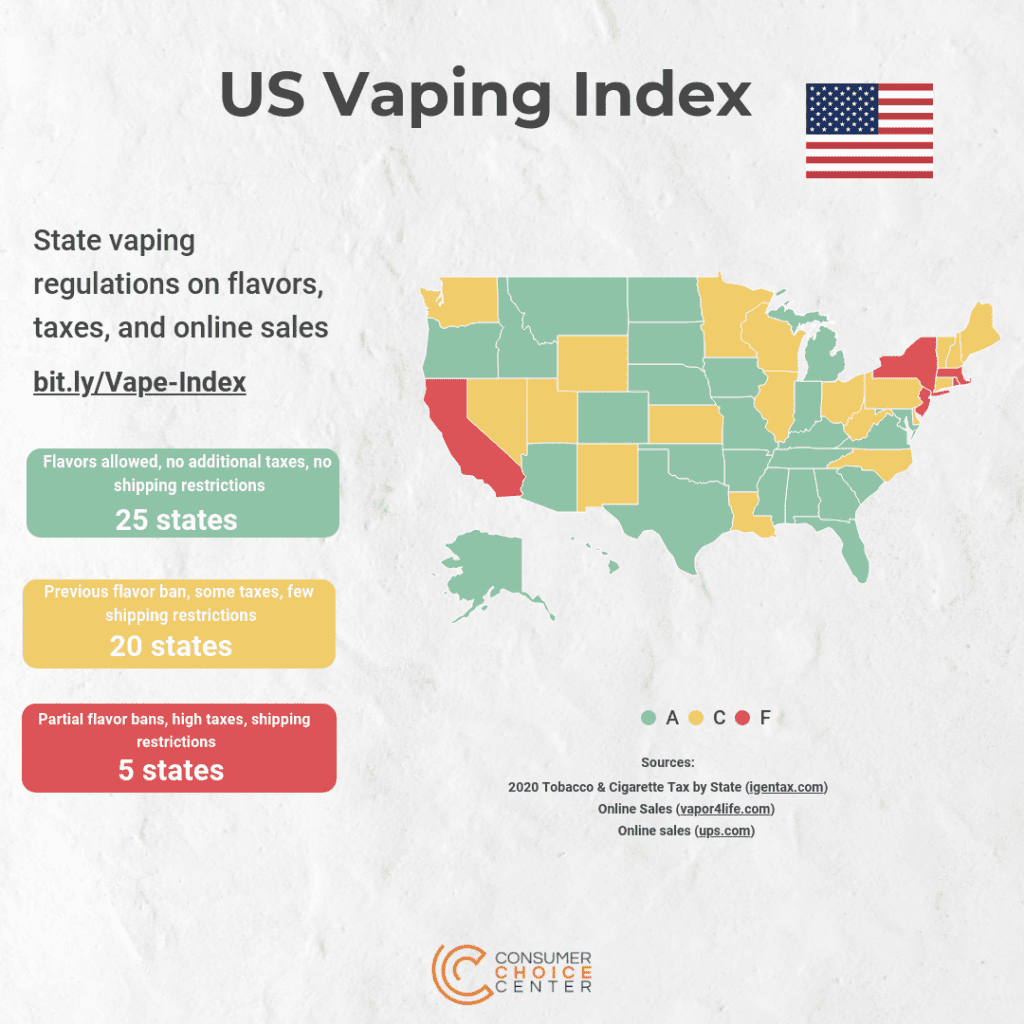
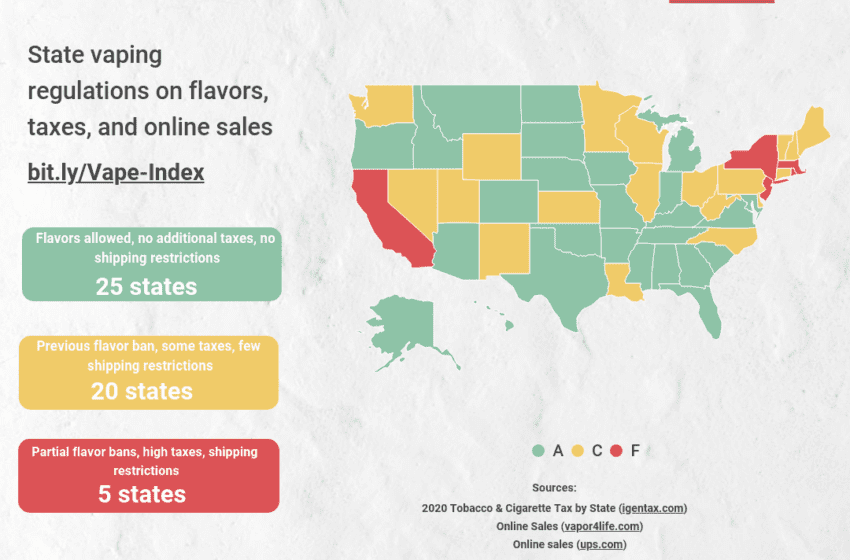
The U.S. Food and Drug Administration (FDA) stated today that it still intends to prioritize enforcement against any electronic nicotine delivery system (ENDS) product that continues to be sold and for which the agency has not received a premarket submission as indicated in FDA’s enforcement priorities guidance.
“Now that the deadline has passed, and the submissions are with FDA, many may be wondering about the upcoming steps for both submitters and the Agency,” the agency wrote in today’s release. “As Mitch Zeller, CTP Director, stated in a recent perspective piece, FDA strives to be as transparent as possible with regards to the status of these submissions and plans to provide regular updates to the public over the course of the next year.”
The factors behind enforcing a device will include several factors, including the likelihood of youth use or initiation. The regulatory agency stated that it will make the best use of agency resources to enforce against any other deemed new tobacco product that does not have the required premarket authorization (PMTA).
in January of this year, the agency did not mention prioritizing open-systems. The three urgencies that earned a bullet-point were:
- “Any flavored, cartridge-based ENDS product (other than a tobacco- or menthol-flavored ENDS product);
- “All other ENDS products for which the manufacturer has failed to take (or is failing to take) adequate measures to prevent minors’ access; and
- “Any ENDS product that is targeted to minors or whose marketing is likely to promote use of ENDS by minors.”
“New data, such as that from the 2020 National Youth Tobacco Survey (NYTS), will also inform the FDA’s enforcement and other actions, and flavored disposable ENDS will be an enforcement priority for the agency,” according to today’s agency release.
The 2020 NYTS showed a large decrease in youth vaping. On Sept. 10, the FDA announced that after two years of disturbing increases in youth e-cigarette use, the agency was “encouraged by the overall significant decline reported in 2020,” the FDA stated in a release. “This is good news; however, the FDA remains very concerned about the 3.6 million U.S. youth who currently use e-cigarettes and we acknowledge there is work that still needs to be done to curb youth use.”
Complicating matters, while the FDA has said that it plans to post a list of the deemed new tobacco products that were on the market in the U.S. as of Aug. 8, 2016, are still on the market now, and for which a premarket submission was made by Sept. 9, 2020, that list may not be available for many weeks or even months.
“Before making such a list available, FDA needs to ensure that publishing any such information complies with federal disclosure laws and regulations,” the FDA wrote. “For example, before FDA can include a specific product on this public list, the Agency may need to verify with companies, on a case-by-case basis, the current marketing status of a product and whether it was on the market as of Aug. 8, 2016.”